Mount Sinai First to Use Visually Guided Catheter Ablation System to Treat Atrial Fibrillation Patient in New U.S. Clinical Trial
The clinical trial is headed by Vivek Y. Reddy, MD, Professor of Cardiology and Director of the Cardiac Arrhythmia Service at Mount Sinai Heart.
For the first time in a new U.S. clinical trial, researchers at Mount Sinai School of Medicine have used the HeartLight Endoscopic Ablation System (EAS) to correct abnormal electrical signals inside the heart of a patient affected by atrial fibrillation (AFib), one of the nation’s most common heart ailments. The device is the first catheter ablation system to incorporate a camera that allows doctors to see a direct, real-time image of the patient’s heart tissue during ablation.
The HeartLight EAS national clinical trial is headed by Vivek Y. Reddy, MD, Professor of Cardiology, Mount Sinai School of Medicine, and Director of the Cardiac Arrhythmia Service at Mount Sinai Heart. Along with colleagues, he performed the successful procedure on the first patient on Valentine’s Day last week. His colleagues include Srinivas R. Dukkipati, MD, Director of Mount Sinai’s Experimental Electrophysiology Laboratory, and Andre d’Avila, MD, Co- Director of the Cardiac Arrhythmia Service.
"This new device has the potential to make AFib ablation more reliable, more reproducible, and more consistent for patients with paroxysmal [intermittent] atrial fibrillation," said Dr. Reddy. "The technology which is currently available leads to widely variable success rates, depending largely on physician skill and experience with the procedure. This visually-guided system with a rotating laser design has the potential to simplify AFib ablation and make it available to more patients than ever, before their paroxysmal AFib becomes chronic [continuous] AFib."
Estimates are that there are as many as six million U.S. adults with atrial fibrillation (AFib), a condition characterized by a rapid and irregular heart beat that can cause serious complications, including stroke, palpitations, fainting and early death. AFib diagnosis has increased over the past two decades and the condition now accounts for one-quarter of all strokes in the elderly.
Paroxysmal (intermittent) AFib is caused by irregular electrical signals that come from pulmonary veins that drain blood from the lungs to the heart. During a standard AFib ablation procedure, physicians use spot catheters to cauterize heart tissue in a point-by-point manner to encircle these pulmonary veins, creating a ring of scar tissue that electrically isolates the pulmonary veins, preventing the irregular electrical signals from causing AFib. But this is a technically complicated procedure since, unlike open heart surgery, physicians cannot directly see the tissue that is being cauterized. Despite using various cardiac mapping systems, problems often arise because the scar tissue that is created is not continuous, allowing the abnormal electrical signals to continue to pass into the heart and cause recurrence of AFib.
The new balloon catheter device used by the Mount Sinai Heart team features a built-in camera that allows the physicians to directly see the heart tissue that needs to be ablated. They can then guide an internal laser in a continuous arc around the origin of the vein, creating more uniform scar tissue. "By directly seeing the tissue that we are ablating, there is less chance of a gap in the encircling ablation line," said Dr. Dukkipati.
The trial is expected to eventually enroll an estimated 450 patients at up to 25 sites in the U.S. and follow them for up to 5 years post procedure. The Food and Drug Administration (FDA) granted Investigational Device Exemption approval for HeartLight EAS in December, and the device is already in use in Europe.
More information about the clinical trial can be found at http://clinicaltrials.gov/show/NCT01456000.
Dr. Vivek Reddy is the national PI and a Co-Investigator of this study at Mount Sinai School of Medicine. Dr. Reddy receives financial compensation as a consultant for CardioFocus, Inc., the study sponsor and manufacturer of the endoscopic ablation system evaluated in the study.
About The Mount Sinai Medical Center
The Mount Sinai Medical Center encompasses both The Mount Sinai Hospital and Mount Sinai School of Medicine. Established in 1968, Mount Sinai School of Medicine is one of the leading medical schools in the United States. The Medical School is noted for innovation in education, biomedical research, clinical care delivery, and local and global community service. It has more than 3,400 faculty in 32 departments and 14 research institutes, and ranks among the top 20 medical schools both in National Institutes of Health (NIH) funding and by U.S. News & World Report.
The Mount Sinai Hospital, founded in 1852, is a 1,171-bed tertiary- and quaternary-care teaching facility and one of the nation’s oldest, largest and most-respected voluntary hospitals. In 2011, U.S. News & World Report ranked The Mount Sinai Hospital 16th on its elite Honor Roll of the nation’s top hospitals based on reputation, safety, and other patient-care factors. Of the top 20 hospitals in the United States, Mount Sinai is one of 12 integrated academic medical centers whose medical school ranks among the top 20 in NIH funding and U.S. News & World Report and whose hospital is on the U.S. News & World Report Honor Roll. Nearly 60,000 people were treated at Mount Sinai as inpatients last year, and approximately 560,000 outpatient visits took place.
For more information, visit http://www.mountsinai.org/.
Find Mount Sinai on:
Facebook: http://www.facebook.com/mountsinainyc
Twitter @mountsinainyc
YouTube: http://www.youtube.com/mountsinainy
About the Mount Sinai Health System
Mount Sinai Health System is one of the largest academic medical systems in the New York metro area, with more than 43,000 employees working across eight hospitals, over 400 outpatient practices, nearly 300 labs, a school of nursing, and a leading school of medicine and graduate education. Mount Sinai advances health for all people, everywhere, by taking on the most complex health care challenges of our time — discovering and applying new scientific learning and knowledge; developing safer, more effective treatments; educating the next generation of medical leaders and innovators; and supporting local communities by delivering high-quality care to all who need it.
Through the integration of its hospitals, labs, and schools, Mount Sinai offers comprehensive health care solutions from birth through geriatrics, leveraging innovative approaches such as artificial intelligence and informatics while keeping patients’ medical and emotional needs at the center of all treatment. The Health System includes approximately 7,300 primary and specialty care physicians; 13 joint-venture outpatient surgery centers throughout the five boroughs of New York City, Westchester, Long Island, and Florida; and more than 30 affiliated community health centers. We are consistently ranked by U.S. News & World Report's Best Hospitals, receiving high "Honor Roll" status, and are highly ranked: No. 1 in Geriatrics and top 20 in Cardiology/Heart Surgery, Diabetes/Endocrinology, Gastroenterology/GI Surgery, Neurology/Neurosurgery, Orthopedics, Pulmonology/Lung Surgery, Rehabilitation, and Urology. New York Eye and Ear Infirmary of Mount Sinai is ranked No. 12 in Ophthalmology. U.S. News & World Report’s “Best Children’s Hospitals” ranks Mount Sinai Kravis Children's Hospital among the country’s best in several pediatric specialties.
For more information, visit https://www.mountsinai.org or find Mount Sinai on Facebook, Twitter and YouTube.
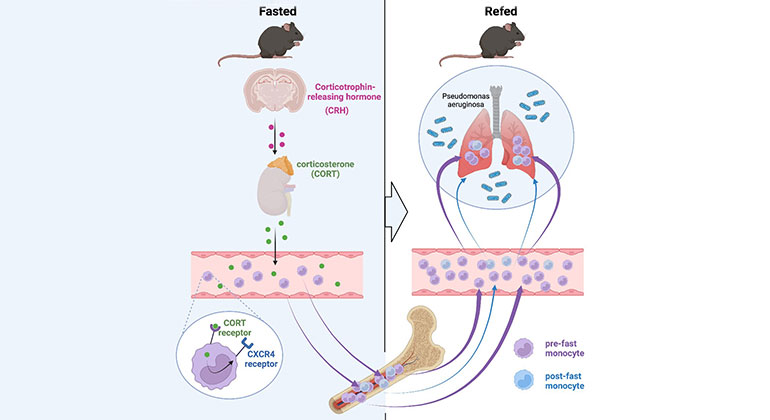
Skipping Breakfast May Compromise the Immune System
Feb 23, 2023 View All Press Releases
Valentin Fuster, MD, PhD, Receives Prestigious Award from City of Barcelona, Spain
Jan 23, 2023 View All Press Releases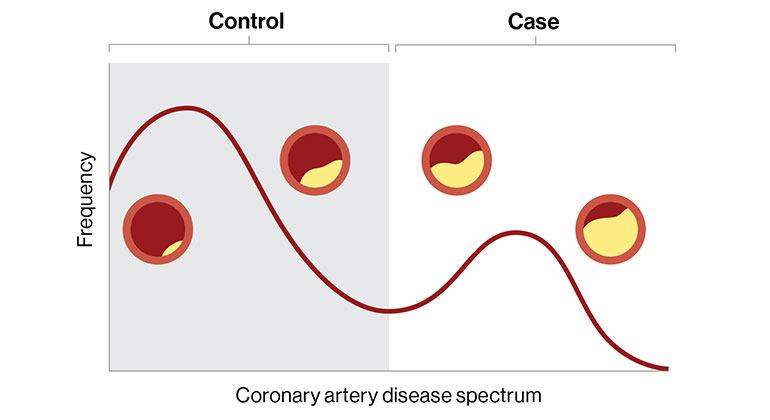
Digital Marker for Coronary Artery Disease Built by Researchers at Mount Sinai
Dec 20, 2022 View All Press Releases
Samin Sharma, MD, Named Director of the Mount Sinai Cardiovascular Clinical Institute
Nov 28, 2022 View All Press Releases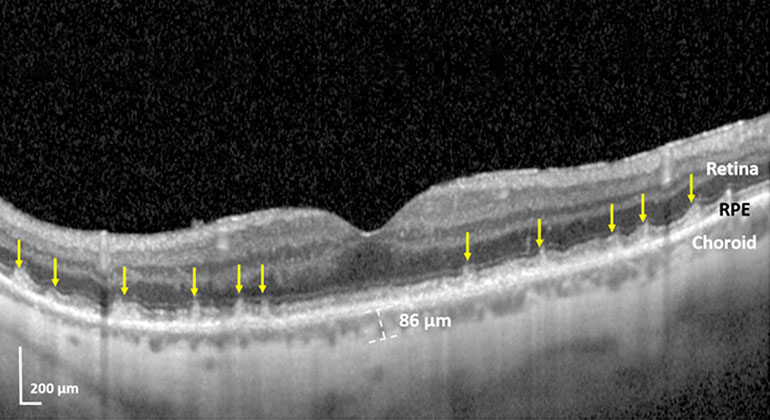
Blinding Eye Disease Strongly Associated With Serious Forms of Cardiovascular Disease
Nov 17, 2022 View All Press Releases




