Early Clinical Trial Tests Immune-Boosting Therapy Before Prostate Cancer Surgery
Presurgery treatment appeared safe and showed signs of triggering immune activity
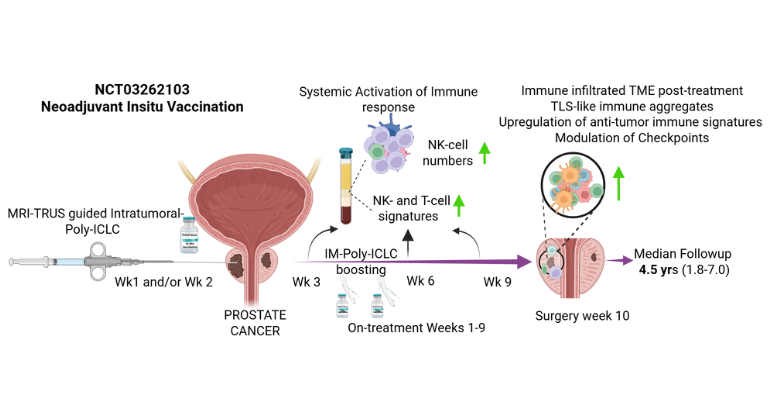
A novel pre-surgery therapy triggered immune responses in prostate tumors and produced encouraging early tumor changes, offering a potential new path for immunotherapy. Image credit: Nair et al., Med
A small, early clinical trial led by the Icahn School of Medicine at Mount Sinai and collaborators has shown that directly injecting an immune-activating compound into prostate tumors before surgery appears safe and may help the immune system recognize and attack cancer cells.
Published in the October 30 online issue of Med [https://doi.org/10.1016/j.medj.2025.100879], a Cell Press journal, the phase I study (NCT03262103) tested a viral-mimicking drug called poly-ICLC in 12 men with intermediate- to high-risk prostate cancer. The neoadjuvant (presurgery) treatment was delivered in two doses—first into the tumor to trigger an immune response, then into a muscle to boost it. The goal was to train the immune system to recognize the cancer before it was removed.
Using imaging to guide the injections, researchers delivered the drug into the tumor and then tracked changes in tissue and blood. The therapy was well tolerated and appeared to jump-start immune activity—drawing immune cells into the tumor, shifting gene patterns, and creating small pockets of immune cells where none had been before, say the investigators.
“Our findings provide an important proof of concept that the immune system in prostate cancer can be reawakened,” says physician-scientist Ash Tewari, MD, MBBS, MCh, who led the phase 1 trial and is senior and corresponding author. Dr. Tewari is the Kyung Hyun Kim, MD Professor and System Chair of the Milton and Carroll Petrie Department of Urology at the Icahn School of Medicine. “By delivering poly-ICLC straight into the tumor under MRI–ultrasound fusion guidance, we were able to engage local immunity before surgery. The same approach could be used to inject other immune agents or combinations, opening new paths for targeted treatment in the prostate. If larger studies confirm these results, this tumor-focused ‘autovaccination’ strategy could become an innovative way to make immunotherapy more effective for men with aggressive prostate cancer.”
In addition to showing signs of immune activation, several patients demonstrated favorable early pathology changes following treatment. While the study’s size limits conclusions about long-term outcomes, the findings suggest this strategy could help reprogram “cold” tumors into “immune-active” ones, reported the investigators.
“High-risk prostate cancer often returns after treatment and has been largely resistant to immunotherapy because the tumors don’t naturally trigger strong immune responses,” says lead and corresponding author Sujit S. Nair, PhD, Assistant Professor and Director of Genitourinary Immunotherapy Research in the Department of Urology at the Icahn School of Medicine. “We wanted to test whether we could safely ‘warm up’ these tumors by activating the immune system before surgery, and the early signals are encouraging. To our knowledge, this is the first prostate cancer trial to test intratumoral immunotherapy. Our method doesn’t rely on a single tumor target and trains the immune system to recognize the whole tumor,” he added.
The results provide a basis for testing how this approach might be combined with standard or experimental immunotherapies in future trials.
“The idea of transforming a tumor into its own vaccine by locally stimulating immune recognition is one of the most exciting directions in cancer research,” says corresponding author Nina Bhardwaj, MD, PhD, Ward-Coleman Chair in Cancer Research, and Director of the Vaccine and Cell Therapy Laboratory at the Icahn School of Medicine. “This work shows that even tumors once thought to be invisible to the immune system can potentially be made responsive.”
Next, the research team plans to move forward with larger, controlled phase 2 clinical trials to test clinical benefit and explore combination strategies with hormone therapies or other immunotherapies. They will also study how this approach reshapes the tumor–immune relationship over time and whether it can help identify which patients are most likely to benefit.
The paper is titled “Prostate cancer in situ autovaccination with the intratumoral viral mimic poly-ICLC: Modulating the cold tumor microenvironment.”
The study’s authors, as listed in the journal, are Sujit S. Nair, Dimple Chakravarty, Sreekumar Balan, Alexander Hakansson, Manuel Duval, Elai Davicioni, Yang Liu, Swati Bhardwaj, Tin Htwe Thin, Monica Garcia-Barros, Kenneth Haines, Majd Al Shaarani, Rachel Weil, Marcia Meseck, Parita Ratnani, Monali Fatterpekar, Elena Gonzalez-Gugel, Adam Farkas, Vinayak Wagaskar, Ivan Jambor, Kacie Schlussel, Cristina Pasat-karasik, Kamala Bhatt, Zachary Dovey, Adriana Pedraza, Akriti Gupta, Dara Lundon, Ante Peros, Sneha Parekh, Lily Davenport, Xiangfu Zhang, Raghav Gupta, Macy Robison, Cynthia Knauer, Ethan Ellis, Dmitry Rykunov, Boris Reva, Babu Padanilam, Matthew D. Galsky, Rachel Brody, Mani Menon, Andres M. Salazar, Nina Bhardwaj, and Ashutosh K. Tewari.
The work was funded by the Arthur M. Blank Family Foundation. See the journal paper for details on conflicts of interest: [https://doi.org/10.1016/j.medj.2025.100879].
About the Icahn School of Medicine at Mount Sinai
The Icahn School of Medicine at Mount Sinai is internationally renowned for its outstanding research, educational, and clinical care programs. It is the sole academic partner for the seven member hospitals* of the Mount Sinai Health System, one of the largest academic health systems in the United States, providing care to New York City’s large and diverse patient population.
The Icahn School of Medicine at Mount Sinai offers highly competitive MD, PhD, MD-PhD, and master’s degree programs, with enrollment of more than 1,200 students. It has the largest graduate medical education program in the country, with more than 2,600 clinical residents and fellows training throughout the Health System. Its Graduate School of Biomedical Sciences offers 13 degree-granting programs, conducts innovative basic and translational research, and trains more than 560 postdoctoral research fellows.
Ranked 11th nationwide in National Institutes of Health (NIH) funding, the Icahn School of Medicine at Mount Sinai is among the 99th percentile in research dollars per investigator according to the Association of American Medical Colleges. More than 4,500 scientists, educators, and clinicians work within and across dozens of academic departments and multidisciplinary institutes with an emphasis on translational research and therapeutics. Through Mount Sinai Innovation Partners (MSIP), the Health System facilitates the real-world application and commercialization of medical breakthroughs made at Mount Sinai.
-------------------------------------------------------
* Mount Sinai Health System member hospitals: The Mount Sinai Hospital; Mount Sinai Brooklyn; Mount Sinai Morningside; Mount Sinai Queens; Mount Sinai South Nassau; Mount Sinai West; and New York Eye and Ear Infirmary of Mount Sinai
About the Mount Sinai Health System
Mount Sinai Health System is one of the largest academic medical systems in the New York metro area, with 48,000 employees working across seven hospitals, more than 400 outpatient practices, more than 600 research and clinical labs, a school of nursing, and a leading school of medicine and graduate education. Mount Sinai advances health for all people, everywhere, by taking on the most complex health care challenges of our time—discovering and applying new scientific learning and knowledge; developing safer, more effective treatments; educating the next generation of medical leaders and innovators; and supporting local communities by delivering high-quality care to all who need it.
Through the integration of its hospitals, labs, and schools, Mount Sinai offers comprehensive health care solutions from birth through geriatrics, leveraging innovative approaches such as artificial intelligence and informatics while keeping patients’ medical and emotional needs at the center of all treatment. The Health System includes approximately 9,000 primary and specialty care physicians and 10 free-standing joint-venture centers throughout the five boroughs of New York City, Westchester, Long Island, and Florida. Hospitals within the System are consistently ranked by Newsweek’s® “The World’s Best Smart Hospitals, Best in State Hospitals, World Best Hospitals and Best Specialty Hospitals” and by U.S. News & World Report's® “Best Hospitals” and “Best Children’s Hospitals.” The Mount Sinai Hospital is on the U.S. News & World Report® “Best Hospitals” Honor Roll for 2025-2026.
For more information, visit https://www.mountsinai.org or find Mount Sinai on Facebook, Instagram, LinkedIn, X, and YouTube.
New AI Tool by Mount Sinai Researchers Could Reshape Prostate Cancer Care
Sep 23, 2024 View All Press ReleasesMount Sinai and Man Cave Health Launch Sports-Themed Resource Center
Jan 02, 2019 View All Press Releases


