Dr. C. Warren Olanow: “FDA Clears Enteral Suspension (Duopa) for Parkinson's”
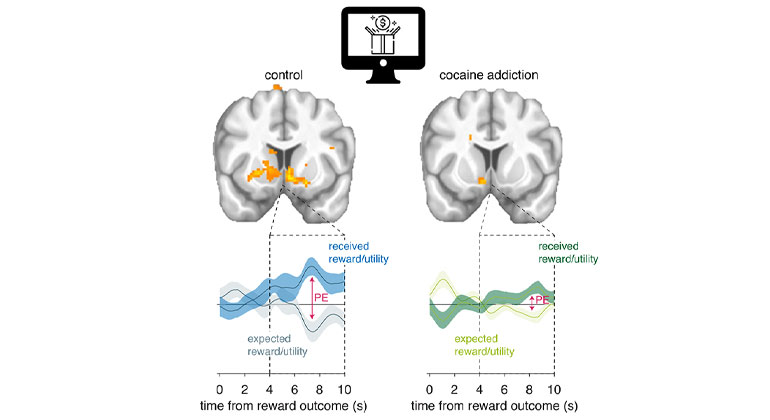
The US Food and Drug Administration (FDA) has approved carbidopa/levodopa enteral suspension (Duopa, AbbVie) for the treatment of motor fluctuations in patients with advanced Parkinson's disease, according to a company news release. The approval was based on a 12-week, phase 3, double-blind, multicenter trial that compared the efficacy and safety of the enteral suspension with those of oral, immediate-release (IR) carbidopa-levodopa tablets in 71 patients with advanced PD. The trial was led by C. Warren Olanow, MD, professor, Department of Neurology and Department of Neuroscience, the Icahn School of Medicine at Mount Sinai, New York, New York. Learn more
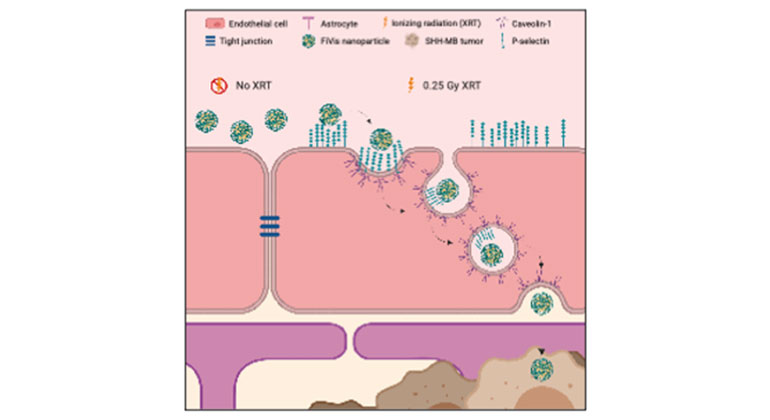
Scientists Develop Novel Approach to Enhance Drug Delivery for Brain Tumors in Children
Mar 02, 2023 View All Press Releases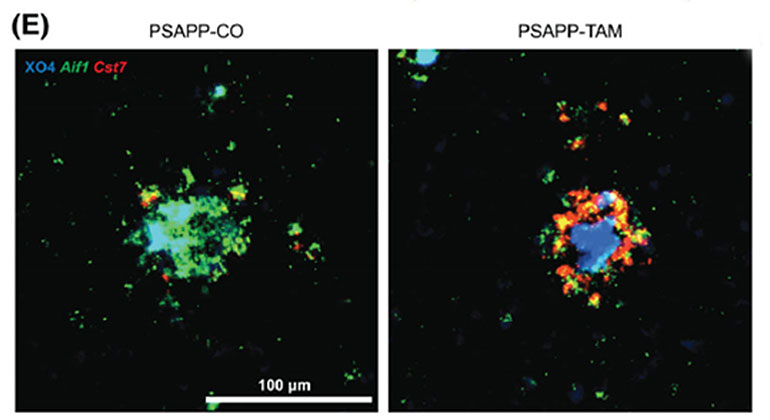
Researchers Identify the Role of an Alzheimer’s Disease Risk Gene in the Brain
Nov 30, 2022 View All Press Releases