Mount Sinai First in U.S. to Use New Drug-Coated Balloon Catheter
Prakash Krishnan, MD, FACC was the first doctor in the United States to use the new drug-coated balloon for peripheral arterial disease (PAD).
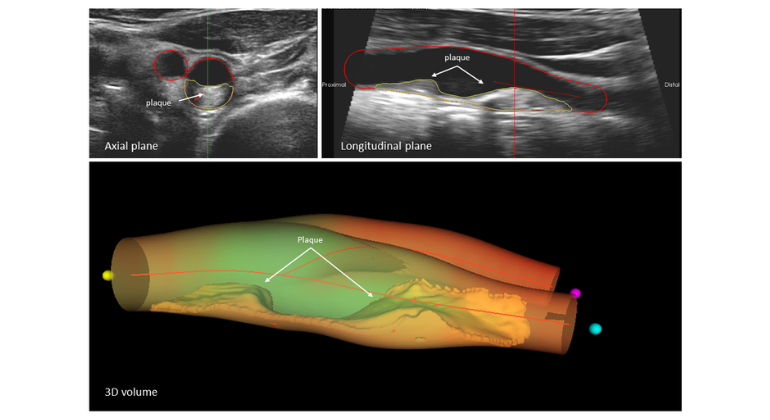
The Mount Sinai Hospital is first in the United States to use the first and only FDA-approved, drug-coated balloon to open blocked arteries in the leg.
"I am excited to be first in providing this FDA approved technology to our peripheral arterial disease patients at The Mount Sinai Hospital," says Prakash Krishnan, MD, FACC, Director of Endovascular Services at Mount Sinai Heart who performed the first case post-FDA approval on October 11 with Jose Wiley, MD, FACC, and Bhaskar Purushottam, MD, of Mount Sinai. "This drug coated balloon may be a game changer in the management of patients with peripheral arterial disease. It can effectively reduce the recurrence of blockages in patients, which can help prevent limb amputations, improve a patient's quality of life, and also be lifesaving."
The device is used during an angioplasty procedure in the Cardiac Catheterization Laboratory for patients with peripheral arterial disease who have severely blocked femoro-popliteal arteries, major arteries that supply blood throughout the legs. The innovative balloon works to dilate a blocked artery restoring blood flow to the limb, while administering a therapeutic dose of the drug paclitaxel inside the artery to prevent it from narrowing in the future.
PAD is a life-threatening condition affecting at least 8 million Americans. The condition narrows arteries reducing blood flow to the limbs. Patients with PAD in their femoropopliteal arteries, especially those older than 50 years of age and those with diabetes, are at increased risk for lower-extremity amputation.
The new device – the Lutonix® 035 Drug Coated Balloon (DCB) Catheter – is made by C.R. Bard, Inc., and received FDA approval on October 10.
"Preventing future artery blockages is as important as opening the initial blockage," says Dr. Krishnan. "This drug-coated balloon technology can help prevent further blockage in the artery."
Mount Sinai was one of 54 sites in the world participating in the LEVANT 2 pivotal study, a global randomized clinical trial that compared the new drug-coated balloon to the current standard therapy of using a non-drug coated balloon catheter during angioplasty. After one year, the study results, which led to the device's FDA approval, demonstrated the technology improved blood flow in arteries, increased patient mobility for walking longer distances, and reduced the rates of blood clots.
Dr. Krishnan served as principal investigator at Mount Sinai for the LEVANT 2 pivotal study. He also is a consultant for C.R. Bard, Inc. the maker of the drug-coated balloon catheter.
About the Mount Sinai Health System
Mount Sinai Health System is one of the largest academic medical systems in the New York metro area, with 48,000 employees working across seven hospitals, more than 400 outpatient practices, more than 600 research and clinical labs, a school of nursing, and a leading school of medicine and graduate education. Mount Sinai advances health for all people, everywhere, by taking on the most complex health care challenges of our time—discovering and applying new scientific learning and knowledge; developing safer, more effective treatments; educating the next generation of medical leaders and innovators; and supporting local communities by delivering high-quality care to all who need it.
Through the integration of its hospitals, labs, and schools, Mount Sinai offers comprehensive health care solutions from birth through geriatrics, leveraging innovative approaches such as artificial intelligence and informatics while keeping patients’ medical and emotional needs at the center of all treatment. The Health System includes approximately 9,000 primary and specialty care physicians and 11 free-standing joint-venture centers throughout the five boroughs of New York City, Westchester, Long Island, and Florida. Hospitals within the System are consistently ranked by Newsweek’s® “The World’s Best Smart Hospitals, Best in State Hospitals, World Best Hospitals and Best Specialty Hospitals” and by U.S. News & World Report's® “Best Hospitals” and “Best Children’s Hospitals.” The Mount Sinai Hospital is on the U.S. News & World Report® “Best Hospitals” Honor Roll for 2024-2025.
For more information, visit https://www.mountsinai.org or find Mount Sinai on Facebook, Instagram, LinkedIn, X, and YouTube.
Mount Sinai Surgeons Perform First Heart-Liver-Kidney Transplants in New York State
May 20, 2025 View All Press Releases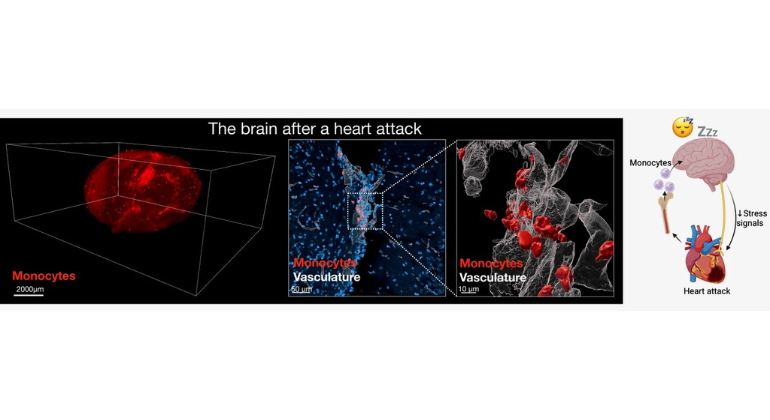
After a Heart Attack, the Heart Signals to the Brain to Increase Sleep to Promote Healing
Oct 30, 2024 View All Press Releases