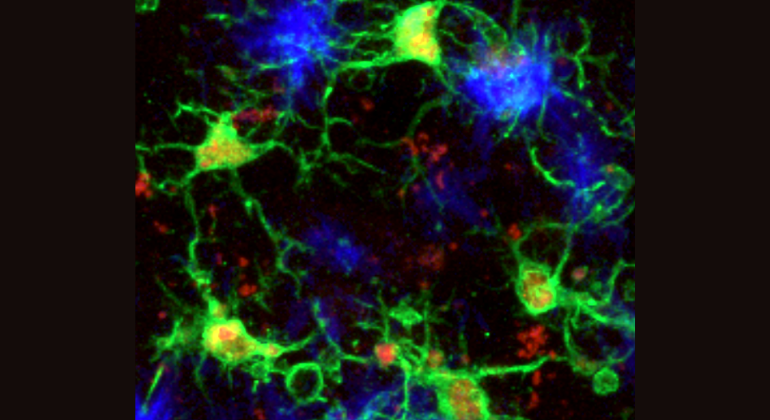
Mount Sinai Researchers Unveil New Targets for Depression at Neuroscience 2012
Scientists presented important discoveries on the involvement of the immune system and dopamine on the onset of depression.
Researchers from Mount Sinai School of Medicine presented important discoveries on the involvement of the immune system and dopamine cells in the onset of depression at Neuroscience 2012, the Society for Neuroscience's 42nd annual meeting on October 13 -17 in New Orleans.
In addition to scientists presenting at the conference, Dennis S. Charney, MD, Anne and Joel Ehrenkranz Dean, Mount Sinai School of Medicine, Executive Vice President for Academic Affairs, The Mount Sinai Medical Center, is available to speak about depression and the psychobiological mechanisms of human resistance to stress. As a renowned expert in the neurobiology and treatment of mood and anxiety disorders, he has led research teams that have discovered that ketamine is a rapidly-acting drug in patients with treatment-resistant depression.
Dr. Charney has co-authored a book, "Resilience: The Science of Mastering Life's Greatest Challenges," and a recent review article on this topic is featured in this week's issue of the journal Science, found here. The website also includes a podcast on how resilience training may help as a treatment for depression.
Highlights of Mount Sinai research at Neuroscience 2012:
---Inhibiting Dopamine Reduces Symptoms of Depression in an Animal Model
Led by Dipesh Chaudhury, PhD, Associate Scientist, and Jessica Walsh, MSc, in the laboratory of Ming-Hu Han, PhD, in the Department of Pharmacology and Systems Therapeutics at Mount Sinai, researchers evaluated the activity of dopamine neurons in the ventral tegmental area, the brain's reward center. These neurons produce the brain's dopamine, a chemical that regulates reward-seeking behavior.
To assess whether inhibiting dopamine neurons affected depression, the research team modulated dopamine cell activity in mice using a novel technique called optogenetics. Optogenetics allows scientists to engineer molecules that can be targeted to specific neurons, in this case dopamine neurons, and increase or decrease their activity using light.
By increasing the activity of dopamine neurons in already-stressed mice, the researchers produced depressed behavior. Inhibiting the activity of dopamine neurons in depressed mice eased their depression.
"Increasing evidence shows that depression may not only be caused by a chemical imbalance in the brain but also by neuronal misfiring in different areas brain," said Dr. Chaudhury. "By targeting neurons in the reward center of the brain, we were able to demonstrate that this chemical may be a promising target for new treatments for depression."
---Innate Immune System Influences Vulnerability to Anxiety or Depression in Animal Model
Scientists have hypothesized that depression results from an inflammatory response in the brain, indicating some immune system involvement. Led by Georgia Hodes, PhD, a Postdoctoral Fellow in the laboratory of Scott Russo, PhD, in the Department of Neuroscience at Mount Sinai, a team of researchers tested this hypothesis by evaluating a cytokine called interleukin-6, which is a protein released by white blood cells in response to injury and is found in elevated levels in people with treatment-resistant depression. They found no evidence of interleukin-6 being made in the brain areas examined, suggesting that it is released in the peripheral immune system.
Next, the Mount Sinai team transplanted the bone marrow of depressed mice into healthy mice and found that these previously healthy mice exhibited signs of depression after experiencing a mild stressor. They also found that mice with immune cells that release more interleukin-6 in response to a toxin developed a more severe depression-like response to the stress.
"To our knowledge, this is the first study showing a functional role for the peripheral immune system in an animal model of depression," said Dr. Hodes. "This study suggests that cytokine-based antibody therapy currently approved for treatment of inflammatory illnesses such as rheumatoid arthritis and Castleman's disease in humans may have potential as an antidepressant treatment."
Other researchers involved in Dr. Hodes work include Miriam Merad, PhD, Marilyn Lebouef, MS, and graduate students Madeline Pfau, Daniel Christoffel, Sam Golden, and MD/PhD student Mitra Heshmati.
The Mount Sinai Medical Center is one of the world's leading institutions in discovering better ways to prevent, diagnose and treat serious brain diseases. Mount Sinai's Department of Neuroscience ranks number five in the nation for National Institutes of Health (NIH) funding, and its work in multiple sclerosis (MS) has been recognized with more than $44 million in NIH research grants—the largest amount ever awarded by NIH for MS.
The Mount Sinai Medical Center is one of just five locations in the United States using new combined MRI/PET technology to image the brain with an unprecedented level of precision to find more effective interventions for addiction, Alzheimer's disease, depression, multiple sclerosis, and other debilitating diseases.
Dr. Chaudhury's research was supported by the National Institute of Mental Health and a Johnson & Johnson/IMHRO Rising Star Translational Research Award.
Dr. Hodes' research was supported by the National Institute of Mental Health, Johnson & Johnson/IMHRO Rising Star Translational Research Award, and an institutional training grant from the National Institute on Drug Abuse.
Abstracts
Innate peripheral immune responses predispose mice to susceptibility to repeated social defeat stress.
Georgia E. Hodes*, Sam A. Golden, Daniel J. Christoffel, Madeline Pfau, Mitra Heshmati, Brandon L. Warren, Carlos A. Bolaños-Guzmán, Scott J. Russo.
1Fishberg Department of Neuroscience and Freidman Brain Institute, Mount Sinai School of Medicine, NY, NY, 10029, USA.
2Psychology and Program in Neuroscience, Florida State University, Tallahassee, FL 32306
The pro-inflammatory cytokine Interleukin-6 (IL-6) is increased in the blood of subjects with major depressive disorder (Dowlati et al., 2009). It is currently unknown whether peripheral IL-6 levels are altered as a result of a depressive episode or whether innate differences in the immune response to stress are functionally involved in the etiology of depression. We utilized repeated social defeat stress (RSDS), a mouse model of depression, to examine the functional relevance of peripheral IL-6 expression. This model allows for the examination of individual differences in responsivity to the stressor; some animals termed susceptible show a spectrum of depression-like behaviors, whereas resilient animals behave more akin to controls. A time-course analysis of peripheral blood IL-6 indicated that prior to the development of any depression-like phenotype, susceptible mice exhibit heightened IL-6 levels following their first exposure to an aggressor, which remain elevated 48 hours after completion of RSDS. Additionally, emotional stress generated by watching RSDS combined with social isolation also significantly increased IL-6 levels in blood compared to control animals, suggesting that this is not exclusively a consequence of more physical trauma in susceptible mice. We next examined whether innate differences in peripheral IL-6 expression contributed to these individual differences. Peripheral mononuclear cells (PBMCs) were isolated from animals prior to defeats and stimulated with lipopolysaccharide (LPS) to examine innate immune response. PBMCs from animals who later showed a susceptible phenotype following RSDS had an exaggerated release of IL-6 following the LPS stimulation. In vivo, we were able to block susceptibility to RSDS by systemically injecting an antibody that neutralized IL-6 in the periphery. Together these studies indicate that innate differences in the inflammatory response to stress underlie the development of depression like behavior in the social defeat model. NIMH 1R01MH090264 ; NIDA 5TDA07135-28
Optogenetic dissection of the functional role of the firing patterns of ventral tegmental area dopamine neurons in encoding behavioral susceptibility to social defeat stress.
*D. Chaudhury, J. J. Walsh, B. Juarez, A. K. Friedman, J. Koo, D. Ferguson, H.-C. Tsai, L. Pomeranz, S. Ku, D. J. Christoffel, E. Mouzon, M. Lobo, R. L. Neve, J. M. Friedman, S. Russo, K. Deisseroth, E. J. Nestler, M.-H. Han; Pharmacol. and Therapeut., Mount Sinai Sch. Of Med., New York, NY; Stanford Univ., Stanford, CA; Rockefeller Univ., New York, NY; Univ. of Maryland, Baltimore, MD; MIT, Cambridge, MA
The role of high frequency phasic firing of ventral tegmental area (VTA) dopamine (DA) neurons in mediating stress vulnerability is not completely understood. In a chronic social defeat model of depression, our recent studies found that mice exhibiting a susceptible (depressive), but not resilient (non-depressive) phenotype, exhibited consistently increased phasic firing of VTA DA neurons. To investigate the casual relationship between phasic firing in these neurons and susceptibility to social defeat in freely-behaving mice, we selectively targeted DA cells by injecting a Cre-dependent viral vector AAV-DIO-ChR2 (channel rhodopsin2), into the VTA of transgenic TH-Cre mice. First, through in vitro and in vivo electrophysiological recordings, we demonstrated that light activation of ChR2 reliably generated physiologically relevant low frequency tonic and high frequency phasic firing patterns in VTA DA neurons. We then showed that optogenetic induction of phasic, but not tonic, firing, in VTA DA neurons of mice, during a social interaction test, 24 hours after undergoing a subthreshold social defeat paradigm, induced a susceptible phenotype as measured by increased social avoidance and decreased sucrose preference (anhedonia). Furthermore, optogenetic phasic stimulation of previously resilient mice induced the susceptible phenotype. To further explore the importance of neuronal firing in modulating behavioral susceptibility to social defeat stress we are performing ‘rescue' experiments. Here, we are utilizing the Cre-dependent viral vector AAV-DIO-NpHR (Halorhodopsin 3.0) to decrease the activity of VTA DA neurons of previously susceptible mice in order to promote the resilient phenotype. These studies provide direct evidence showing that the phasic firing pattern of VTA DA neurons in the brain reward circuitry encodes a signal for stress vulnerability.
About The Mount Sinai Medical Center
The Mount Sinai Medical Center encompasses both The Mount Sinai Hospital and Mount Sinai School of Medicine. Established in 1968, Mount Sinai School of Medicine is one of the leading medical schools in the United States. The Medical School is noted for innovation in education, biomedical research, clinical care delivery, and local and global community service. It has more than 3,400 faculty in 32 departments and 14 research institutes, and ranks among the top 20 medical schools both in National Institutes of Health (NIH) funding and by US News and World Report.
The Mount Sinai Hospital, founded in 1852, is a 1,171-bed tertiary- and quaternary-care teaching facility and one of the nation's oldest, largest and most-respected voluntary hospitals. In 2011, US News and World Report ranked The Mount Sinai Hospital 14th on its elite Honor Roll of the nation's top hospitals based on reputation, safety, and other patient-care factors. Mount Sinai is one of 12 integrated academic medical centers whose medical school ranks among the top 20 in NIH funding and US News and World Report and whose hospital is on the US News and World Report Honor Roll. Nearly 60,000 people were treated at Mount Sinai as inpatients last year, and approximately 560,000 outpatient visits took place.
For more information, visit http://www.mountsinai.org.
Find Mount Sinai on:
Facebook: http://www.facebook.com/mountsinainyc
Twitter: @mountsinainyc
YouTube: http://www.youtube.com/mountsinainy
###
About the Mount Sinai Health System
Mount Sinai Health System is one of the largest academic medical systems in the New York metro area, with 48,000 employees working across seven hospitals, more than 400 outpatient practices, more than 600 research and clinical labs, a school of nursing, and a leading school of medicine and graduate education. Mount Sinai advances health for all people, everywhere, by taking on the most complex health care challenges of our time—discovering and applying new scientific learning and knowledge; developing safer, more effective treatments; educating the next generation of medical leaders and innovators; and supporting local communities by delivering high-quality care to all who need it.
Through the integration of its hospitals, labs, and schools, Mount Sinai offers comprehensive health care solutions from birth through geriatrics, leveraging innovative approaches such as artificial intelligence and informatics while keeping patients’ medical and emotional needs at the center of all treatment. The Health System includes approximately 9,000 primary and specialty care physicians and 10 free-standing joint-venture centers throughout the five boroughs of New York City, Westchester, Long Island, and Florida. Hospitals within the System are consistently ranked by Newsweek’s® “The World’s Best Smart Hospitals, Best in State Hospitals, World Best Hospitals and Best Specialty Hospitals” and by U.S. News & World Report's® “Best Hospitals” and “Best Children’s Hospitals.” The Mount Sinai Hospital is on the U.S. News & World Report® “Best Hospitals” Honor Roll for 2025-2026.
For more information, visit https://www.mountsinai.org or find Mount Sinai on Facebook, Instagram, LinkedIn, X, and YouTube.
Mount Sinai Study Discovers Potential Link Between Stress and Type 2 Diabetes
Sep 03, 2025 View All Press Releases