Kravis Children’s Hospital at Mount Sinai
Kravis Children’s Hospital at Mount Sinai is the clinical home of Mount Sinai’s Jack and Lucy Clark Department of Pediatrics, and we share a common vision to improve the health of infants, children, adolescents, and young adults through prevention, early detection of disease, and evidence-based treatment, with an emphasis placed on quality and safety of care.
Department of Pediatrics
Recognized as among the top children’s hospitals in several specialties, Mount Sinai has created an alliance with the Children’s Hospital of Philadelphia, one of the nation’s leading hospitals, to give children in the New York metropolitan area access to an unprecedented scope of pediatric services with access to the newest treatments and expertise in fetal medicine, pediatric cardiac care, and pediatric oncology.
This report highlights:
- Peter Pastuszko, MD, renowned pediatric cardiac surgeon, who has joined Mount Sinai
- Defining a genetic framework for inflammatory bowel disease
- A pioneering treatment for Batten’s disease
- A rare neoplasm, surgery, and novel intraperitoneal therapy
- Research on asthma and infectious disease
- A complex case of aHUS
Renowned Pediatric Cardiac Surgeon Joins Mount Sinai
Peter Pastuszko, MD, who has extensive experience in treating the entire spectrum of congenital heart disease—from newborn to adult, having performed several thousand successful surgeries over the course of his career—recently joined the Mount Sinai Health System as Chief of Pediatric Cardiac Surgery and Director of Pediatric Cardiovascular Services.
He will also play a significant role in the new alliance in pediatrics between the Mount Sinai Health System and Children’s Hospital of Philadelphia. The alliance combines the strengths of both institutions, includes a Fetal Heart Program, and offers joint expertise in the diagnosis and treatment of congenital heart disease from prenatal diagnosis through adulthood. Dr. Pastuszko trained at Children’s Hospital of Philadelphia under the mentorship of Thomas L. Spray, MD, Chief of the Division of Cardiothoracic Surgery, an internationally recognized leader in his field.
Dr. Pastuszko, who also has Senior Faculty appointments in the departments of Cardiovascular Surgery, and Pediatrics, at the Icahn School of Medicine at Mount Sinai, has expertise in the surgical treatment of complex neonatal heart defects, including hypoplastic left heart syndrome (HLHS), transposition of great arteries (TGA), truncus arteriosus, and Ebstein’s anomaly, among others. Dr. Pastuszko has performed, with a high success rate, the Norwood operation, neonatal Ross procedure, and neonatal Ebstein’s repair, and is also experienced in the area of adult congenital, thoracic, and vascular surgery.
Additionally, he has been recognized by national and international cardiothoracic societies as one of the foremost researchers on the protection of the newborn brain from hypoxia before, during, and after surgery. His investigative efforts, in collaboration with researchers at the University of Pennsylvania and University of California, San Diego, are focused on protective pharmacological interventions. Dr. Pastuszko has authored several dozen research publications, as well as multiple abstracts and book chapters on the subject.
Defining a Genetic Framework for Inflammatory Bowel Disease
Researchers have identified more than 200 inflammatory bowel disease (IBD) risk loci, revealing overlap between ulcerative colitis (UC) and Crohn’s disease (CD). It is thought that a patient’s susceptibility loci signature may give insight into the molecular basis of a sub-phenotype of IBD. Already, genetics-driven therapy can be seen in action in the Very Early Onset IBD (VEO-IBD) population, where monogenic diseases are more common and some variants require lifesaving bone marrow transplantation.
At Kravis Children’s Hospital at Mount Sinai, Marla C. Dubinsky, MD, Chief, Pediatric Gastroenterology and Hepatology, and Elizabeth Spencer, MD, PGY-6, sought to create an IBD gene panel to target four subsets of genes.
They are:
- genes for VEO-IBD
- genes for prognostication, for example, NOD2
- genes that can direct, or potentially direct, therapy, for example, thiopurine methyltransferase (TPMT), one of the most important enzymes in the metabolism of thiopurines (6-mercaptopurine and azathiopurine), and IL23R, significant because the newest CD therapy, ustekinumab, targets IL12 and IL23 through their common p40 subunit
- genes implicated in medically refractory disease.
The team first identified the panel of genes after conferring with world experts in VEO-IBD and IBD genetics. The selected genes were curated, and the evidence associating each gene with IBD was manually assessed and quantified based on a known variant classification scale, and later cataloged with specific IBD phenotype descriptors.
Drs. Dubinsky and Spencer noted significant overlap with primary immunodeficiency (PID) panels, and they are currently curating a sister PID panel, which will be validated with known patients who have variants by Sema4, a newly created Mount Sinai Health System genome-based diagnostic venture, and sent for approval to New York State.
A Pioneering Treatment for Batten’s Disease
In January 2016, a 3-year-old boy presented to the pediatric emergency room at Mount Sinai Beth Israel with jerking movements and recent language regression—symptoms that mimicked those of his 16-year-old sister, who is completely dependent in all activities of daily life and living in a residential facility. An immediate 48-hour video EEG revealed seizures.
He was evaluated by Steven Wolf, MD, and Patty McGoldrick, NP, MPA, at the hospital’s Developmental Disability Center, who suspected neuronal ceroid lipofuscinosis type 2 (CLN2) also known as tripeptidyl peptidase 1 (TPP1) deficiency—Batten’s disease. The rare and rapidly progressive disease, which also afflicts his sister, is caused by the absence or reduced activity of the TPP1 enzyme. This results in the accumulation of lysosomal storage materials, leading to neuronal degeneration and neuronal death. Testing revealed that he was positive for CLN2.
Historically, there has been no treatment for Batten’s disease. However, in April 2017, the U.S. Food and Drug Administration approved an enzyme replacement therapy, known as Brineura™, developed by BioMarin, in which cerliponase alfa (rhTTP1), a proenzyme, is taken up by target cells in the central nervous system and is translocated to the lysosomes.
After the child met multiple criteria involving psychological, speech, occupational, and physical therapy evaluations—efforts coordinated by the departments of Neurosurgery, Pediatrics, Neurology, Genetics and Genomic Sciences, and Social Work Services—placement of an Ommaya reservoir for intrathecal medication administration was performed by Saadi Ghatan, MD, in June 2017. One month later, Amy Yang, MD, performed the intraventricular cerliponase alfa injection at Kravis Children’s Hospital at Mount Sinai—the first center in the Northeast to provide this treatment.
As of December 5, 2017, he has undergone nine infusions, which act to prevent worsening gait abnormalities. The child has a new school setting with therapies, durable medical equipment, and home care services. He continues to be cared for by Dr. Wolf; Ms. McGoldrick; Dr. Yang; Beth Brown, LCSW; and developmental pediatrician, Soledad Guzman, MD. It is their hope that this child will fare better than his sister.
Steven Wolf, MD, is Director of Pediatric Epilepsy at the Mount Sinai Health System, the Comprehensive Pediatric Epilepsy Center, and the Developmental Disability Center; Patty McGoldrick, NP, MPA, is Associate Director of the Developmental Disability Center and Co-Director of the Comprehensive Pediatric Epilepsy Center; Amy Yang, MD, is Assistant Professor of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai; and Saadi Ghatan, MD, is the Director of Pediatric Neurosurgery, Mount Sinai Health System, and Chair of Neurosurgery at Mount Sinai West and Mount Sinai St. Luke’s.
A Rare Neoplasm, Surgery, and Novel Intraperitoneal Therapy
In April 2017, a 10-year-old male patient was referred to the Kravis Children’s Hospital at Mount Sinai for a rapidly growing tumor in his abdomen that impaired his ability to eat, walk, or participate in any other normal childhood activity. He underwent a tumor biopsy and drainage of ascites by Peter Midulla, MD, Associate Professor of Surgery, and Pediatrics, and Chief of Pediatric Surgery. The patient was diagnosed with desmoplastic small round cell tumor (DSRCT), a rare and aggressive malignant neoplasm that had spread to the patient’s liver, spleen, bones, and lungs in addition to the large abdominal mass.
Because the tumor at diagnosis was inoperable, the patient was referred for chemotherapy to Birte Wistinghausen, MD, Associate Professor of Pediatrics (Hematology-Oncology); and Pamela Merola, MD, Assistant Professor of Pediatrics.
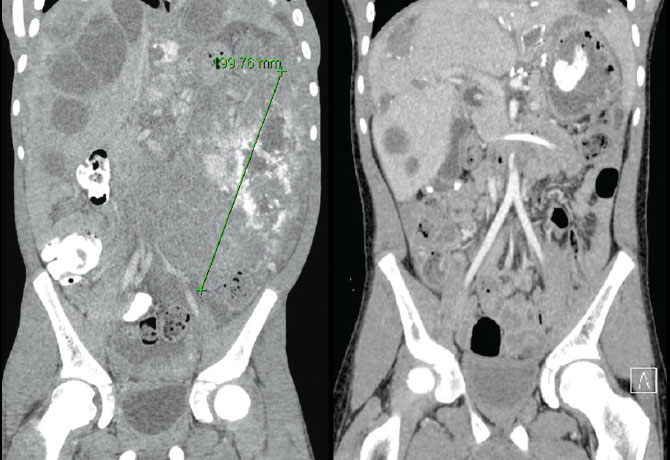
He tolerated six months of intensive chemotherapy well, with a great clinical and functional response allowing him again to eat, walk, and participate in most age-appropriate activities. Repeat imaging showed resolution of the lung disease and evidence of good response in his abdomen, liver, spleen, and bones. However, he required resection of the residual abdominal tumors that were partially calcified and studding his entire abdominal cavity.
In a very complex surgical procedure lasting 14 hours, the masses were removed by Brian Coakley, MD, Assistant Professor of Surgery; and Daniel Labow, MD, Chair, Department of Surgery, Mount Sinai St. Luke’s and Mount Sinai West. During the surgery, he underwent a novel technique, hyperthermic intraperitoneal chemoperfusion, which delivers heated chemotherapy directly into the abdominal cavity, where it can penetrate the diseased tissue directly.
This procedure has been used in adults with extensive abdominal tumors, but only recently in children with DSRCT. Mount Sinai is one of two centers in the country to offer this potentially lifesaving procedure. The patient tolerated the procedure well, but developed an inflammatory response leading to a buildup of fluid surrounding his heart that was relieved by interventional pediatric cardiologist Barry Love, MD, Assistant Professor of Pediatrics, and Medicine, who placed a pericardial drain. The patient has since recovered well and is in excellent clinical shape, and ready to complete his treatment with further chemotherapy and radiation therapy.
Is Asthma an Infectious Disease?
It is well known that asthma exacerbations can be triggered by transient exposures to environmental allergens, including fungi. Until recently, actual infection with fungi has not been considered to be an important contributor in the pathophysiology of disease. Obtaining evidence has been difficult, as routine culture techniques have proven insufficient to diagnose such infection in the lower airways.
However, Alfin G. Vicencio, MD, Vice Chair for Clinical Affairs and Strategy and Division Chief for Pediatric Pulmonology at Kravis Children’s Hospital at Mount Sinai, with collaborators at the Albert Einstein College of Medicine—by employing newer molecular techniques, specifically mycobiome analysis, to increase the sensitivity of detection—recently uncovered evidence that may challenge this concept.
Although the investigators had a goal to confirm the presence of commonly implicated fungi, such as Aspergillus and Alternaria, in the lower airways of children with severe asthma, they were intrigued to find a different organism that had not previously been implicated.
In a letter recently published in The Journal of Allergy and Clinical Immunology, the authors demonstrated for the first time that Pneumocystis jirovecii is present in bronchoalveolar lavage fluid from approximately 80 percent of the specimens they obtained from children with severe asthma, compared to no presence in non-asthma patients.
While these results are preliminary and require additional investigation, a research group at the University of Pittsburgh used serologic markers to obtain similar evidence of Pneumocystis jirovecii infection in adult patients with asthma.
Subtle Findings Point to a Complex Case of aHUS
In June 2017, a 6-month-old female was transferred to Kravis Children’s Hospital at Mount Sinai from a regional tertiary care medical center with severe cardiac dysfunction following in-center cardiopulmonary arrest with successful resuscitation. With a history of two to three months of worsening emesis followed by progressive weight loss, she had been initially admitted with severe hyponatremia, hypokalemia, and anemia (Hb 5.5); she was also found to have severe hypertension.

of therapy, the team noted subtle but key findings in addition to the new and mild AKI and anemia: hypertension, proteinuria, thrombocytopenia, and a low C3 complement level. Atypical hemolytic uremic syndrome (aHUS) was suspected as a root cause of illness, and, while other multisystem diseases with potential overlap were being evaluated, empiric treatment with eculizumab was initiated.
Over time, each of the complications improved, with complete normalization of cardiac and pulmonary function, stabilized neurological status, and most slowly, hematological resolution. Laboratory and genetic studies also further strengthened the diagnosis of aHUS. Recognized for its international expertise in aHUS, a rare disease affecting only several hundred individuals in the United States, the Kravis Children’s Hospital nephrology team foresees, with ongoing treatment, an excellent prognosis for this patient.
Message from the Chair: Lisa M. Satlin, MD
 The Department of Pediatrics continued on a trajectory of significant growth and success in our academic missions of clinical care, research, and education in 2017. Among our accomplishments:
The Department of Pediatrics continued on a trajectory of significant growth and success in our academic missions of clinical care, research, and education in 2017. Among our accomplishments:
- Recognition of the Kravis Children’s Hospital at Mount Sinai by U.S. News & World Report in its 2017-18 “America’s Best Children’s Hospitals” guidebook, with rankings in Diabetes & Endocrinology, Gastroenterology & GI Surgery, Nephrology, Neurology & Neurosurgery, Pulmonology, and Urology.
- Launch of the Mount Sinai–Children’s Hospital of Philadelphia alliance’s Children’s Heart Center, and the creation of a new Pediatric Hospital Medicine Program, and Food Allergy Treatment and Research Center.
- Continued strength in our extramural funding portfolio to more than $23 million in 2017, with top 15 ranking for National Institutes of Health funding for Departments of Pediatrics in U.S. medical schools and No. 1 in New York State (Blue Ridge Institute for Medical Research).
We also are pleased to announce these recent appointments to the Department of Pediatrics:
- John C. Bucuvalas, MD, Chief of the Division of Hepatology, and Vice Chair of Faculty Affairs
- Steven J. Burakoff, MD, Chief of the Division of Hematology-Oncology, as he continues as Dean for Cancer Innovation at the Icahn School of Medicine at Mount Sinai and the Lillian and Henry M. Stratton Professor of Cancer Medicine
- George Ofori-Amanfo, MD, Chief of the Division of Critical Care.
Building on the momentum generated by this past year’s accomplishments, we look forward—as the Icahn School of Medicine at Mount Sinai celebrates its 50th anniversary—to a new year of exciting innovation and discovery focused on improving children’s health.
Lisa M. Satlin, MD, is the Herbert H. Lehman Professor and Chair, Jack and Lucy Clark Department of Pediatrics, and Pediatrician-in-Chief, Kravis Children’s Hospital at Mount Sinai