Departments of Neurology and Neurosurgery
We are dedicated to rapid advances in technology, pioneering research, and multidisciplinary collaboration to set a new standard for team care to dramatically improve the quality of life for patients
Departments of Neurology and Neurosurgery
The Mount Sinai Health System’s departments of Neurology and Neurosurgery are excited about building and integrating our clinical programs across New York City—and throughout the region, nation, and world—and bringing new advances in neuroscience into clinical practice.
This specialty report highlights:
- A multicenter trial in the United States, led by Mount Sinai, to evaluate the performance of Gliolan® (5-ALA) for fluorescence-guided surgery of brain tumors
- Mount Sinai is only one of a few sites in North America to use a specific combination of integrated neurological tools for preoperative simulation, intraoperative navigation, and microscope integration, along with 3D printing, to treat brain tumors
- The development of a novel technique for a devastating form of stroke called Stereotactic ICH Underwater Blood Aspiration (SCUBA) that is offering promising results in minimally invasive hematoma evacuation
- A stereo-EEG technique, a minimally invasive approach to recording a three-dimensional map of the seizure onset and propagation in the brain, that has been successfully treating pediatric epilepsy that is refractory to standard management
- Mount Sinai clinician-scientists pioneering the investigation of a new imaging agent used with PET to detect and monitor the progression of repetitive traumatic brain injury in patients with a history of concussion
Studying Fluorescence-Guided Surgery:
A Multicenter Trial Under Way for Malignant Gliomas
In June 2016, a new investigator-initiated trial—titled A Multicenter Study of 5-Aminolevulinic Acid (5-ALA) to Enhance Visualization of Malignant Tumor in Patients with Newly Diagnosed or Recurrent Malignant Gliomas: A Safety, Histopathology, and Correlative Biomarker Study— began enrolling patients in the Mount Sinai Health System.
This is the first multicenter study in North America utilizing 5-ALA, the active substance in Gliolan® for fluorescence-guided surgery (FGS) of brain tumors. The study is sponsored by Constantinos G. Hadjipanayis, MD, PhD, Professor of Neurosurgery, and Oncological Sciences; Director of Neurosurgical Oncology at the Mount Sinai Health System; and Chair of Neurosurgery at Mount Sinai Beth Israel. He was the first in the United States to perform FGS of a brain tumor after Gliolan administration and is the U.S. Food and Drug Administration (FDA) Investigational New Drug holder. Isabelle M. Germano, MD, Professor of Neurosurgery, Neurology, and Oncological Sciences, and Director of the Comprehensive Brain Tumor Program, is the principal investigator.
Approximately 10 to 20 centers will take part in this study, which will include up to 100 new or recurrent malignant glioma patients. The Henry Ford Health System in Detroit, the first site to be added, enrolled its first patient in January. A correlative serum biomarker study will be included for participating centers.
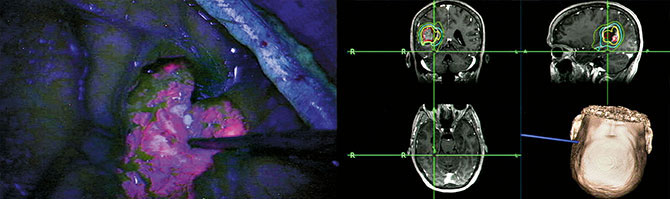
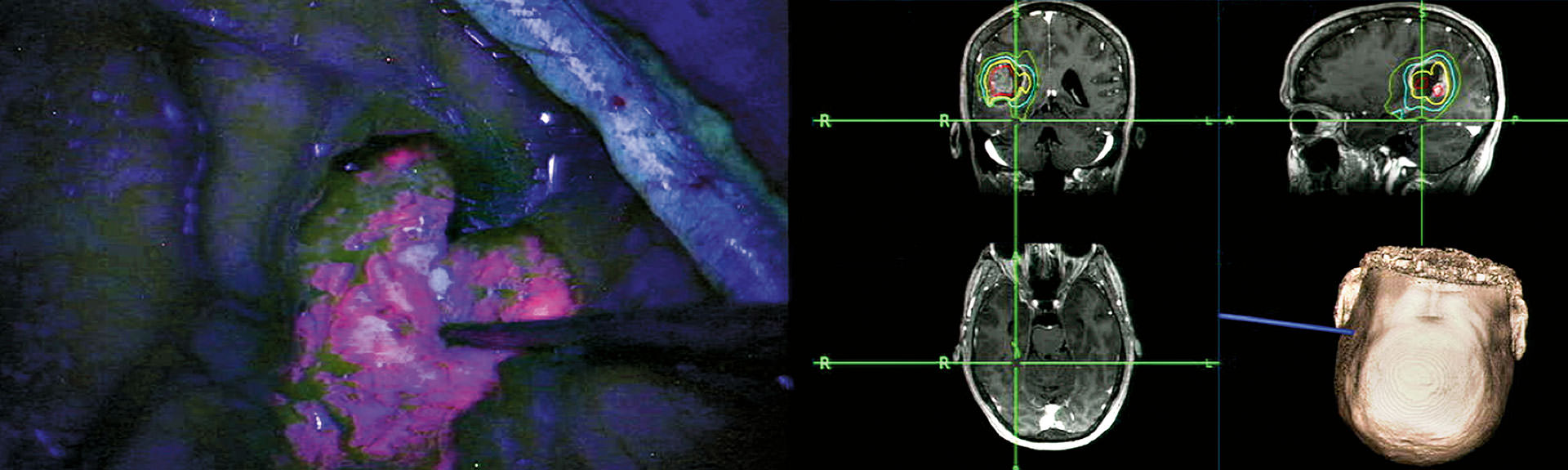
Gliolan FGS has emerged as an important technique for the safe and effective removal of brain tumors by direct visualization of tumor tissue after using a special blue light in the operating room (see cover images). Gliolan is orally administered to patients and is rapidly taken up into the bloodstream, where it penetrates the blood-brain barrier and is taken up by brain tumors. Once it enters brain tumor cells, it is metabolized to its fluorescent form, known as protoporphyrin IX (PPIX). PPIX is visualized as violet-red in tumor tissue with use of a special operative microscope. Better visualization of tumor tissue after 5-ALA administration permits more complete resection of brain tumors and overall benefit to the patient (see images above). This has been shown in a large, randomized study in Europe.
Gliolan FGS is currently approved for brain tumor removal in Europe and Asia, and safety has been demonstrated in many patients. The multicenter trial at Mount Sinai will evaluate the utility of Gliolan, and its metabolite PPIX, as an intraoperative fluorescent detection agent for the visualization of malignant tumor during the removal of malignant brain tumors, either new or recurrent.
Recently, Dr. Hadjipanayis completed a National Institutes of Health (NIH)-funded R21 study on the use of 5-ALA FGS in combination with new whole-brain proton spectroscopic imaging in brain tumor patients. This novel study could lead the way to better identification of malignant brain tumor tissue and radical resection of tumors. Currently, Dr. Hadjipanayis and Daniel J. Brat, MD, PhD, Vice Chair, Translational Programs, Department of Pathology and Laboratory Medicine, Emory University School of Medicine, are studying the expression of proteins in different regions of brain cancer, known as glioblastoma (GBM). By utilizing 5-ALA FGS, Dr. Hadjipanayis and his team can differentiate the tumor margin, where fluorescence is weaker, from the tumor mass. This NIH-funded R01 study aims to provide important information on protein expression at the tumor margin so potential drug therapies can target this problematic area. Nearly all GBM tumors in patients relapse at the tumor margin due to cancer cells that reside away from the tumor mass and escape treatment.
Dr. Hadjipanayis and his team have been integral to the development of Gliolan in the United States over the last six years. Gliolan received orphan drug status from the FDA in 2013 and is now being considered for fast-track FDA approval.
Dr. Hadjipanayis has a financial interest related to the development of Gliolan, which is currently a non-FDA approved agent. Dr. Germano has been appointed as Global PI to oversee Gliolan related research and publication of this work.
Integrated Neurosurgical Tools, 3D Printing:
An Epidermoid Tumor in Posterior Fossa Is Removed
Paul, a 39-year-old male, had begun noticing some loss of hearing and trouble with balance. These symptoms were minor at the time, and with his very active lifestyle, Paul dismissed them. But when he and his wife were on vacation in New York City, he noticed a fullness in his right ear. This prompted him to see Eric A. Munzer, DO, Assistant Clinical Professor, Otolaryngology, Icahn School of Medicine at Mount Sinai, who recommended an MRI. The results showed a large, almost 5 cm diameter epidermoid tumor in the posterior fossa, and Paul was immediately referred to Joshua B. Bederson, MD, Professor and Chair of Neurosurgery for the Mount Sinai System, and Clinical Director of the Neurosurgery Simulation Core.
In August 2016, the patient came to see Dr. Bederson and Leslie Schlachter, PA-C. The team confirmed Dr. Munzer’s findings that the tumor was compressing the patient’s brain stem, and they also learned that it surrounded five different cranial nerves (V7, 8, 9, 10, and 11). The effect on the cranial nerves was very evident in the patient’s symptoms, including intermittent choking when drinking liquids. Paul was scheduled for surgery in a month.
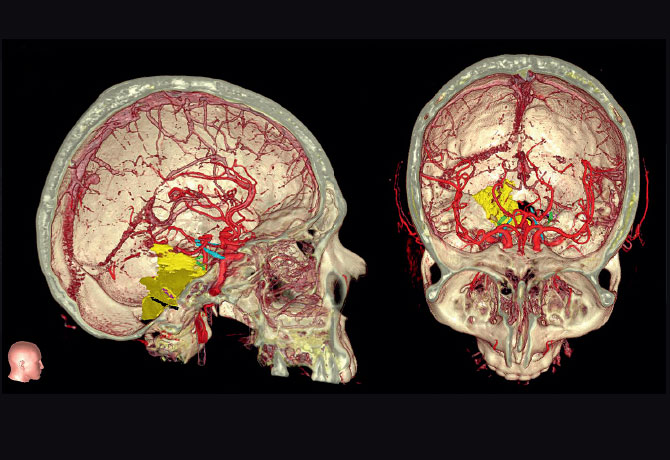
The Mount Sinai team mobilized a specific combination of integrated neurosurgical tools for preoperative simulation, planning, intraoperative navigation, and microscope integration to help Paul. Mount Sinai is one of only a few sites in North America that has this capability. By utilizing Surgical Theater’s patient-specific 3D simulation platform, linked to Brainlab’s surgical navigation hardware and software platforms, which were integrated into the Leica microscope, the surgeon was able to see, in real time, a “heads-up” display of outlined critical structures projected into the eyepieces of the microscope during surgery.
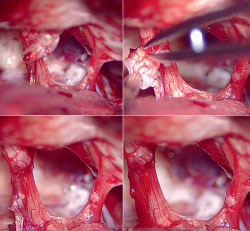
A 3D print of the patient’s anatomy also was made to increase the surgeon’s awareness and provide a lifelike model for preoperative planning and orientation. First, the patient’s MRI and CT data were taken to segment the structures of the skull, tumor, and other important anatomy. The cranial nerves also were segmented and printed, in addition to important vasculature—information that was translated into a multicolor, multistructural gypsum powder print. Under the direction of Anthony B. Costa, PhD, Assistant Professor of Neurosurgery, and Scientific Director of the Neurosurgery Simulation Core, the Medical Modeling Core completed the task of building digital representations of the patient’s anatomy through numerical analysis of preoperative MRI and CT scans. Through the Medical Modeling Core’s collaboration with Mount Sinai’s Rapid Prototyping Center, the final 3D print was completed in about six hours using the in-house 3D Systems’ ProJet® CJP 660Pro full-color 3D printer. This technology allows for vision where traditional imaging methods are not highly contrasting.
The surgical approach was a far-lateral transcondylar craniotomy, allowing for a direct approach to the tumor, avoiding the brain stem. The epidermoid was revealed to be cystic, containing pearls. Removal took several hours of gentle dissection and peeling fibrosis pieces of the tumor carefully from the cranial nerves.
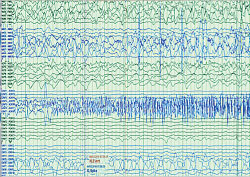
Figure 2: Seizure onset at proximal contacts (nearer to scalp versus deeper in the brain) of the anterior dysembryoplastic neuroepithelial tumor stereo-EEG electrode.
After experiencing some facial numbness, fatigue, and headaches for a few days after surgery, Paul had a speedy recovery. Upon leaving Mount Sinai, he rested in upstate New York with his family for two weeks before he returned to his home in Bermuda. Within a month, he had returned almost to full functioning. At his six-week postoperative checkup with Dr. Bederson, Paul reported that he was at work full time and looking to be active again and playing tennis.
Dr. Bederson owns equity in Surgical Theater, LLC.
Advancing Intracerebral Hemorrhage Care
Making Gains in Minimally Invasive Clot Evacuation
The least treatable and most devastating form of stroke, spontaneous intracerebral hemorrhage (ICH), is a leading cause of mortality, morbidity, and disability worldwide.
Currently, there are very few treatment options for patients with this condition, but recently, minimally invasive clot evacuation has gained popularity as a result of multiple small but successful prospective trials and iterative advances in technology.
J Mocco, MD, MS, Director of the Cerebrovascular Center at the Mount Sinai Health System, and Professor and Vice Chair for Education in the Department of Neurosurgery at the Icahn School of Medicine at Mount Sinai, and Christopher P. Kellner, MD, Director of the Health System’s Intracerebral Hemorrhage program and Assistant Professor of Neurosurgery, have developed an original technique to perform minimally invasive clot evacuation in patients with this condition.
The treatment—Stereotactic ICH Underwater Blood Aspiration technique (SCUBA)—brings together a combination of stereotactic guidance, a low-profile endoscopic sheath, the Apollo™ System device by Penumbra, Inc., for suction with an ultrasonicator, combined air and fluid strategies, and electrocautery. Dr. Mocco, who holds the Investigational Device Exemption for Apollo—approved in 2014 by the U.S. Food and Drug Administration for use in the evacuation of intraventricular hemorrhage and in 2016 for the evacuation of intracerebral hemorrhage—is a co-principal investigator of a multicenter clinical trial known as INVEST: Minimally Invasive Endoscopic Surgery vs. Medical Management in Supratentorial Intraparenchymal Hemorrhage. The INVEST trial is funded by Penumbra, Inc.
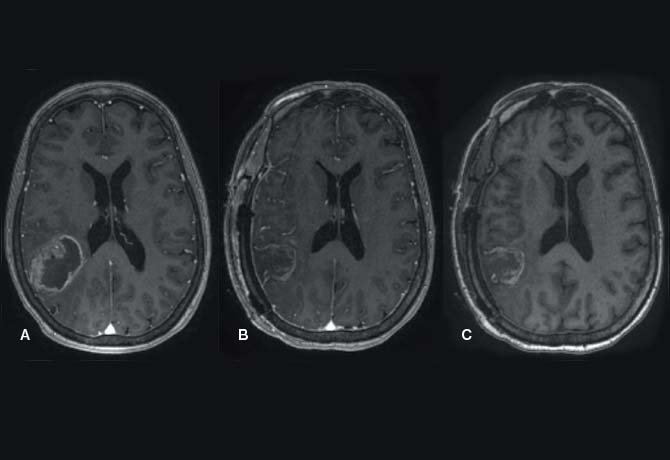
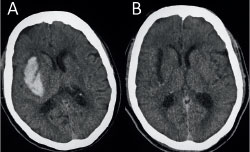
In one patient whom Dr. Mocco and Dr. Kellner treated in 2016, the SCUBA technique was used to achieve a 100 percent evacuation and to cauterize the bleeding artery. The patient was an 81-year-old woman with hypertension and diabetes, who had presented to the Mount Sinai West emergency room with the sudden onset of left hemiplegia, dysarthria, and altered mental status for an NIH Stroke Scale score of 22. She was found to have a right basal ganglia ICH measuring 22 cc (Figure 1A). Forty-eight hours after the bleed occurred, she underwent minimally invasive clot evacuation. A trajectory was planned using stereotactic guidance, and a right frontal burr hole was made. Pre-evacuation ultrasound demonstrated the hematoma. Upon stereotactic placement of the sheath and removal of the introducer, most of the clot expressed itself passively through the sheath.
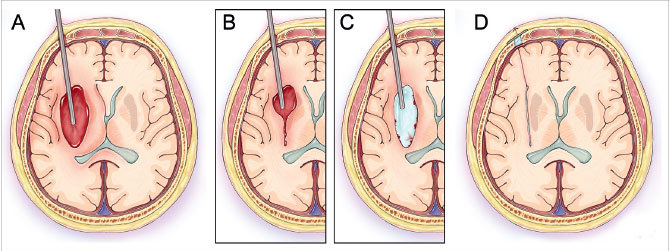
The first phase of the technique consists of aspirating the clot from within the end of the sheath by using light irrigation with the suction strength dialed to 100 percent (Figure 2A). The surgeon aspirates the hematoma until brain material is seen closing in around the end of the sheath. The sheath is then retracted 1 cm so that more hematoma will present itself into the end of the sheath. The process is repeated until the sheath tip reaches the proximal wall of the hematoma (Figure 2B). Irrigation is next increased to permit examination in a fluid-filled cavity for residual clot and/or active bleeding (Figure 2C). In this case, active bleeding was identified and cauterized, and a large piece of residual clot was seen and evacuated. Dr. Mocco and Dr. Kellner evacuated 100 percent of the hematoma (Figures 1B, 2D). The patient improved by 5 NIH Stroke Scale points during hospitalization, and six months later was able to speak normally and walk with a walker, and had good strength in her left arm.
Dr. Mocco has received research grants from Penumbra, Stryker, Microvention, Medtronic, and Codman.
Treating Seizure Disorders:
Combining the Minimally Invasive Stereo-EEG Technique With Awake Language Mapping
The patient is a 15-year-old right-handed young man, diagnosed at age six in his home country with a left temporoparietal and insular dysembryoplastic neuroepithelial tumor (DNET). The tumor was diagnosed after the patient presented with seizures consisting of lifting his right hand above his head and laughing for a few seconds. These seizures occurred approximately every 10 days. His other seizure type consisted of a sensation of an electrical current traveling up through his left leg, which frequently resulted in falls that caused multiple soft-tissue injuries. He was deemed to have gelastic as well as focal seizures without loss of consciousness.
Over the years, in his home country, he tried 15 anti-seizure medications and underwent placement of a vagus nerve stimulator implant—without benefit. His scalp electroencephalogram (EEG) confirmed that the area of onset of his typical seizures was the left temporoparietal area. By functional MRI, language function was determined to reside in the left hemisphere as well. He also underwent an awake craniotomy with a subtotal resection of the tumor. His seizures and speech worsened after the resection, and as sequelae of surgery, he perceived that his hand coordination was decreased on the right.
In February 2016, the patient and his family came to the Mount Sinai Epilepsy Center seeking further help for the seizure disorder. The patient continued to have multiple daily seizures of both the gelastic and the focal motor types. He was determined to be a candidate for evaluation using the stereo-EEG technique, a minimally invasive approach to recording a three-dimensional map of the seizure onset and propagation in the brain. He underwent stereo-EEG placement of eight intracranial electrodes encompassing the tumor area, including anterior, posterior, inferior, and superior, within and in sites distant to the tumor, since DNETs can often cause seizures at locations remote from the lesion itself. With this comprehensive electrode placement, seizure onset and spread patterns within and around the large and deep-seated tumor could be comprehensively, accurately, and safely localized. The procedure was performed without complication.
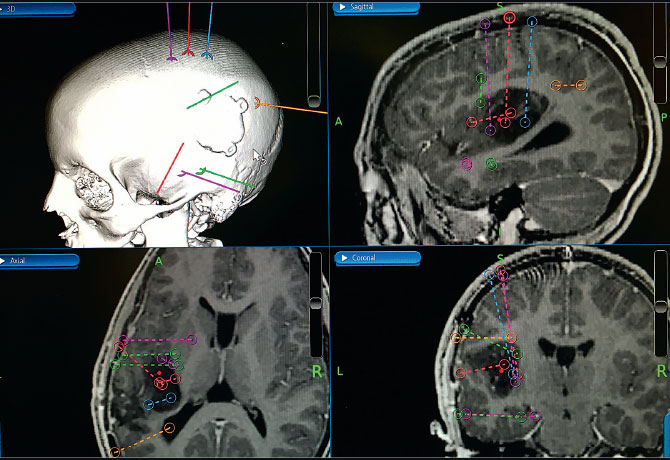
The stereo-EEG implant process involved planning the recording montage as shown in Figure 1, which was based on seizure semiology and scalp EEG recording, as well as on an analysis of neurophysiologic activity around the previous small resection. The stereo-EEG recording clearly showed areas of cortical irritability around the remaining tumor and provided a clear map of seizure onset and spread in the peritumoral cortex. Figure 2 shows the onset of his typical seizure in the proximal (toward the scalp rather than deeper in the brain) stereo-EEG electrode contacts placed anterior to the tumor. Seizure spread occurred to electrode contacts superior to the tumor within five seconds of onset.
This patient presented a unique challenge: he did not speak English and required extensive intraoperative awake language and motor mapping to determine the eloquent and functional cortical areas that would need to be spared during removal of the seizure onset zone. The translation service and anesthesiologists worked closely with the epilepsy team to have at hand several versions of appropriate language tests to be used intraoperatively.
The patient underwent resection of 5 cm3 of his dominant superior temporal gyrus, his seizure onset zone, with mapping of eloquent language and motor areas. He had no deficits after surgery and an immediate cessation of his daily seizures. He returned home within a month, and over the subsequent six months had only rare occurrences of nonepileptic events mimicking seizures, at approximately one per month. He continues to receive his care from an expert epilepsy team in his home country.
The Mount Sinai epilepsy team was led by Saadi Ghatan, MD, Chair of Neurosurgery, Mount Sinai West and Mount Sinai St. Luke’s, and Director of Pediatric Neurosurgery, Mount Sinai Health System; Fedor E. Panov, MD, Assistant Professor of Neurosurgery; Steven M. Wolf, MD, Director of Pediatric Epilepsy and of the Comprehensive Pediatric Epilepsy Center, with Associate Director Patricia Engel McGoldrick, NP, MPA; and Cynthia Harden, MD, Director of Epilepsy Services, Mount Sinai Health System.
Studying a New Imaging Agent:
A Window Into Traumatic Brain Injury in Living Patients
Researchers at the Icahn School of Medicine at Mount Sinai are pioneering the use of a new imaging agent used with positron emission tomography (PET) to detect and monitor the progression of repetitive traumatic brain injury in patients with a history of concussions.
Sam Gandy, MD, PhD, Director of the Center for Cognitive Health and of the National Football League (NFL) Neurological Care Program at the Icahn School of Medicine at Mount Sinai, and Dara L. Dickstein, PhD, Adjunct Assistant Professor of Neuroscience, and Geriatrics and Palliative Medicine, coauthored a proof-of-concept study in Translational Psychiatry in 2016. The new PET agent—a ligand known as T807/AV1451—was used to examine a 39-year-old retired NFL player who had experienced 22 concussions, four of which resulted in loss of consciousness, during an 11-year NFL career.
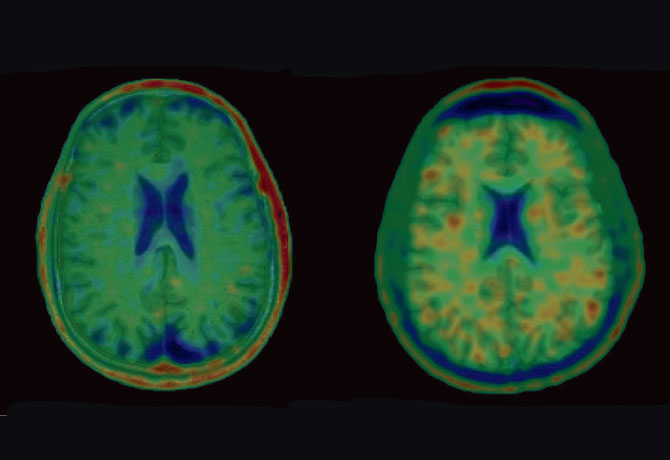
The patient exhibited agitation, impulsivity, sensitivity to light, periods of severe rage, and a decline in executive functioning, processing speed, and fine motor skills, as well as difficulty with working memory—all progressive neuropsychiatric symptoms associated with chronic traumatic encephalopathy (CTE). The disease involves widespread axonal disruption and the eventual degeneration of the neocortex, hippocampus and other limbic structures, and basal forebrain, and is also believed to be a precursor to various neurodegenerative diseases.
Currently, little is known about the progression of CTE pathology in vivo, but with the new ligand, Drs. Gandy and Dickstein were able to discover evidence of CTE in the patient, which appeared as deposits of sticky tau protein in the brain that formed an irregular pattern of clumps rather than a normal pattern of strings. This pattern of ligand binding suggests extensive tauopathy deposition, particularly at the gray-matter/white-matter boundary (see images). Researchers were particularly struck by the apparent similarity of T807/AV1451 retention in this patient with the recent recognition by a National Institute of Neurological Disorders and Stroke CTE neuropathology consensus panel that tauopathy at the depths of the cerebral cortical sulci is pathognomonic for CTE. Furthermore, in January, at the Human Amyloid Imaging Conference in Miami, researchers from Avid
Radiopharmaceuticals, which developed the ligand, reported that the first autopsy case on a CTE patient imaged in life verified that the subcortical ligand retention on the scan matched the distribution of tauopathy.
This case provides a window into the neuropsychiatric and structural progression of CTE and suggests that T807/AV1451 tauopathy imaging is a potentially promising tool to detect CTE-related neuropathology in vivo in at least some patients. Study partners included researchers from Boston University School of Medicine; Emerson Hospital in Concord, Massachusetts; the University of Florida; the University of Virginia; and Weill Cornell Medical College in New York City.
Further research comparing T807/AV1451 results in CTE to healthy controls and other tauopathies will clarify whether there is a signature pattern that can be attributed to CTE and whether and under what conditions tauopathy imaging can be used clinically to identify and track progression of CTE-related neurodegeneration.
The Mount Sinai team is additionally studying 24 patients and plans to establish a clinical trial early this year that will employ the new ligand to identify CTE patients who might respond to an antitauopathy medicine manufactured by Cortice Biosciences, Inc.
Under the leadership of Drs. Gandy and Dickstein, and with funding from the Alzheimer’s Drug Discovery Foundation, Mount Sinai has become one of the few medical centers researching the use of the new ligand. Dr. Dickstein, recently named Associate Professor of
Pathology at the Uniformed Services University of the Health Sciences, in Bethesda, Maryland, will continue to collaborate with Mount Sinai to examine CTE and post-traumatic stress syndrome in battlefield personnel repeatedly exposed to improvised explosive devices.
Message from the Chairs:
Barbara G. Vickrey, MD, MPH
Joshua B. Bederson, MD
Mount Sinai continues to use the most advanced technology to make a lasting, global impact on patient care, research, and outcomes.
For decades, effectively resecting malignant brain tumors has been a challenge. Today, Mount Sinai leads a multicenter trial in the United States to evaluate the performance of Gliolan® (5-ALA) for fluorescence-guided surgery, where the oral amino acid crosses the blood-brain barrier and turns the tumor a violet-red hue and is visualized under blue light.
Intracerebral hemorrhage, the most devastating form of stroke, lacks an effective medical therapy. But with the development of a novel technique called Stereotactic ICH Underwater Blood Aspiration (SCUBA), we are seeing promising results in minimally invasive hematoma evacuation.
Pediatric epilepsy that is refractory to standard management has been successfully treated utilizing a stereo-EEG technique, a minimally invasive approach to recording a three-dimensional map of the seizure onset and propagation in the brain.
Mount Sinai clinician-scientists are pioneering the investigation of a new PET imaging agent to visualize pathological changes in the brain that may signal subsequent decline in individuals who have experienced repeated concussions. After repetitive trauma to the brain, some individuals suffer devastating symptoms of impaired cognition and extreme mood swings. The imaging agent, a ligand, may flag those at risk at an early stage, when interventions can be tested to prevent these long-term effects. When an ongoing preliminary study of 24 patients is complete, Mount Sinai investigators are planning a clinical trial to determine whether this ligand can detect at-risk individuals who have suffered repeated concussions.
Our ability to collaborate and deliver ever higher quality care is made possible by Mount Sinai’s significant investment in research infrastructure and innovative scientists. We are excited about building and integrating our clinical programs across New York City—and throughout the region, nation, and world—and bringing new advances in neuroscience into clinical practice.
Departments of Neurology and Neurosurgery
Download the Winter 2017 Specialty Report