
Division of Nephrology
The Mount Sinai Health System's Division of Nephrology is at the forefront of kidney care and science with expert-led research, cutting-edge clinical innovations, and state-of-the-art facilities.
Division of Nephrology
Mount Sinai Health System’s Division of Nephrology is at the forefront of kidney care and science with expert-led research, cutting-edge clinical innovations, and state of-the-art facilities.
This specialty report highlights:
- A clinical trial led by researchers at the Icahn School of Medicine at Mount Sinai to determine whether an anti-tumor necrosis factor-alpha (TNFa)-blocking antibody can extend the life of a transplanted kidney from a deceased donor
- The Division’s launch of a new unit to research biomarkers for kidney disease and create risk prediction models
- An investigation studying the link between exposure to particulate matter and albuminuria with systematic endothelial dysfunction and increased cardiovascular risk in first responders at the September 11th World Trade Center attacks in 2001
- A comprehensive molecular assay portfolio being developed to monitor kidney function both before and after transplantation in an effort to detect fibrotic events before irreversible damage to the organ occurs
Young Equestrian Is Given the Chance To Compete Again
Rhyan Serres, an energetic and spirited 19-year-old professional equestrian with dreams of being among the elite in her sport, almost lost her life after suffering a severe acute kidney injury as a result of a rare disease.
 The disease, atypical hemolytic uremic syndrome (aHUS), is a progressive, life-threatening illness that frequently has a genetic component. It affects the complement system and the proteins that support the immune system’s ability to use antibodies and phagocytic cells to clear out waste. aHUS is characterized by systematic thrombotic microangiopathy, the formation of micro blood clots in small blood vessels throughout the body, which can damage organs and lead to kidney failure, and has been associated with stroke, heart attack, and death.
The disease, atypical hemolytic uremic syndrome (aHUS), is a progressive, life-threatening illness that frequently has a genetic component. It affects the complement system and the proteins that support the immune system’s ability to use antibodies and phagocytic cells to clear out waste. aHUS is characterized by systematic thrombotic microangiopathy, the formation of micro blood clots in small blood vessels throughout the body, which can damage organs and lead to kidney failure, and has been associated with stroke, heart attack, and death.
Rhyan was treated at a Long Island hospital, where she received hemodialysis, plasmapheresis, and eculizumab, a humanized monoclonal antibody drug that is a terminal complement inhibitor. But she did not respond to treatment and continued to be anuric, anemic, and thrombocytopenic.
Rhyan’s mother sought help from the Division of Nephrology at The Mount Sinai Hospital. In June 2015, Tonia Kim, MD, Assistant Professor of Medicine (Nephrology) at the Icahn School of Medicine at Mount Sinai, received an urgent call for help with the case Rhyan was massively overloaded with fluids—more than 30 pounds over her normal weight. An echocardiogram showed that she also had decreased ejection fraction, suggestive of heart failure. She developed flash pulmonary edema, making it impossible for her to breathe.
She was intubated and immediately transferred to the Medical Intensive Care Unit (MICU) at Mount Sinai and presented with hypertension, oliguria, and anasarca. The nephrology, pulmonary, critical care, and hematology teams had to quickly coordinate and mobilize all efforts to save her life.
The renal ICU team closely orchestrated timely hemodialysis sessions, supplemented with CHF Solutions Aquadex Flexflow ultrafiltration, a nonpharmacological technique that is used to efficiently and carefully remove fluid while maintaining blood pressure and avoiding hypotension. In coordination, the pulmonary critical care team in the MICU was able to closely monitor Rhyan on the ventilator and execute a safe extubation once she was able to breathe unassisted. The hematology team also gave Rhyan eculizumab, the only drug that has proven successful in treating aHUS.
Over three days, 10 liters of fluid were safely removed from her body. She ultimately recovered renal function as measured by creatinine levels in the range of 1. “The renal and ICU teams successfully took a multidisciplinary approach during a life-threatening medical situation and were able to use CHF Solutions to treat a patient with massive fluid overload,” Dr. Kim says. “Our extraordinary critical care team was able to respond quickly and efficiently.”
Rhyan’s kidneys made a remarkable recovery and are back to normal function. Just several weeks after her discharge from Mount Sinai, Rhyan was healthy enough to compete in one of the largest equestrian competitions of her career. She continues to take eculizumab to treat her aHUS and will be carefully monitored by Dr. Kim.
Trial Tests Whether an Antibody Can Protect a Donated Kidney
A multisite clinical trial led by researchers at the Icahn School of Medicine at Mount Sinai will determine whether the life of a transplanted kidney from a deceased donor can be extended by introducing an anti–tumor necrosis factor-alpha (TNFa)-blocking antibody during transplant surgery.
The acute negative effects that can ensue from deceased-donor kidney transplantation include prolonged ischemia followed by reperfusion injury. This complication can result in delayed graft function. Early injury enhances immunogenicity of the organ, subjecting it to late immune-mediated rejection.

Evidence from animal models and some human studies indicates that TNFa acts as a crucial mediator of this early injury by activating T cells and endothelial cells (among other cell types). The clinical trial, which started in October 2015 at 10 sites in the United States and Canada, is led by Peter S. Heeger, MD, Professor of Medicine (Nephrology) and Director of the Translational Transplant Research Center at the Icahn School of Medicine. Dr. Heeger also heads a National Institutes of Health (NIH)–funded consortium—Clinical Trials in Organ Transplantation (CTOT)—which is collaborating on the trial.
To reduce inflammation and enhance immunosuppression, researchers will administer infliximab to half of the 300 trial subjects at the start of transplant surgery, as an addition to the standard of care. Infliximab is an anti-TNFa drug commonly used to treat Crohn’s disease and rheumatoid arthritis.
“The nice thing about the design of this study is that infliximab is a drug that’s given just once yet has the potential to have profound effects for years,” says Dr. Heeger. Currently, the average half-life of a kidney transplant from a live donor is 11 to 14 years, and 10 years if the organ comes from a deceased donor.
“What happens at the very beginning of the transplant likely significantly influences what happens much later,” says Dr. Heeger, who has steered the CTOT, a national leader of multicenter NIH-funded trials, for 11 years. “We’re trying to find the best way to prevent rejection and graft loss without causing complications or side effects.”
The research will require extensive collaboration among the transplant surgeons, nephrologists, nurses, and other staff at the 10 participating sites, which include Yale University School of Medicine; the University of California, San Francisco, School of Medicine; the David Geffen School of Medicine at the University of California, Los Angeles; Emory University School of Medicine; the Cleveland Clinic; University Hospitals Case Medical Center; Washington University School of Medicine in St. Louis; University of Michigan Medical School; and the University of Manitoba College of Medicine. The trial is set to be completed in five years and will include a two-year follow-up on all participants.
Most of the laboratory work for the trial will be conducted at The Mount Sinai Hospital.
For additional information on the clinical trial or to discuss enrolling patients, please call Mount Sinai’s Recanati/Miller Transplantation Institute at 212-731-RMTI (7684).
Research on Biomarkers Aims to Further Earlier Diagnoses and New Therapies
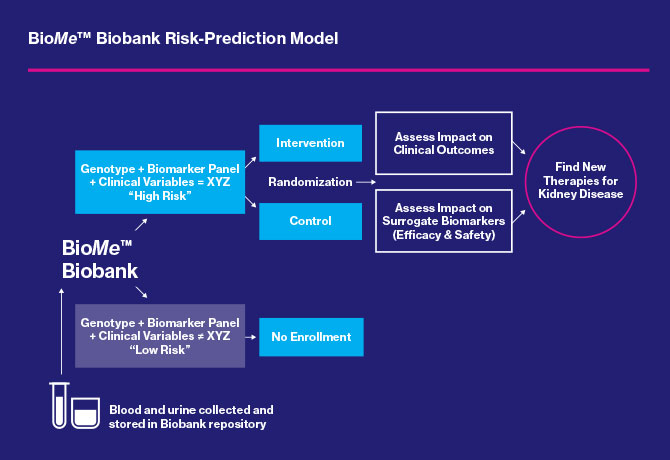
The Division of Nephrology at the Icahn School of Medicine at Mount Sinai has created a new unit to research biomarkers for kidney disease and create risk-prediction models that could lead to earlier diagnoses
as well as new therapies.
Steven Coca, DO, Associate Professor of Medicine (Nephrology), leads the new Clinical and Translational Research Unit (CTRU). By leveraging the Mount Sinai Biobank, known as BioMe™, housed within The Charles Bronfman Institute for Personalized Medicine, Dr. Coca’s team has access to a diverse population of more than 35,000 patients who have consented to DNA sequencing, participation in longitudinal studies related to data embedded in their electronic medical records, and ongoing communication with researchers.

Studies of several biomarkers found in the blood and urine of BioMe participants are enabling the researchers to create risk-prediction models that can lead to more efficient clinical trials.
If the biomarkers are ultimately validated and qualified through the U.S. Food and Drug Administration, they will pave the way for earlier identification of patients with high genetic or phenotypic risk (due to diabetes) who are likely to develop or experience worsening of kidney disease.
“The biobank is very robust because it contains not only genetic data but clinical data as well,” Dr. Coca says. “We have a powerful cohort enabling us to explore more associations of biomarkers and enroll patients most likely to benefit from potential trial interventions based on their clinical, genetic, and biomarker profiles.”
Dr. Coca’s focus is on diabetic kidney disease, and he is studying 1,600 diabetics who are already taking ACE inhibitors or angiotensin receptor blockers. Girish Nadkarni, MD, MPH, Assistant Director of the CTRU, is researching African American and Hispanic patients who carry the APOL1 risk allele, which puts them in danger of developing progressive kidney disease.
Preliminary studies show that the most successful biomarkers for kidney disease may include tumor necrosis factor receptor (TNFR) 1 and TNFR2, which have shown consistently high prognostic ability across race and among both diabetics and non-diabetics.
“These are the biomarkers in which we have the most confidence at this time,” Dr. Coca says. “We’ve been having some success with them for predicting progressive kidney disease months to years after we’ve collected the samples.”
Other than standard treatment using ACE inhibitors, ntil now no therapy has been found to prevent progression of kidney disease.
CTRU researchers are using multiplex technology that measures multiple biomarkers simultaneously on several axes, including inflammation, endothelial injury, kidney tubule injury, and kidney fibrosis. The innovative technology allows for more rapid acquisition of data and significantly reduces costs.
“The beauty is, the BioMe biobank is composed of Mount Sinai patients who are still in the database. As a result, high-risk patients can be enrolled in the study, and participants most in need of intervention can be identified more quickly than if vast numbers of patients from clinics needed to be screened. Employment of appropriate biomarkers for kidney-disease screening may help reduce the length of trials or the number of study participants by 30 to 50 percent,” Dr. Coca says.
Unique Kidney Study Investigates the Impact of 9/11 Exposure on First Responders
Two Mount Sinai physicians are investigating an association between the intensity of exposure to particulate matter and albuminuria (a marker of early chronic kidney disease, or CKD), systematic endothelial dysfunction, and increased cardiovascular risk in a cohort of first responders at the World Trade Center (WTC) attacks on September 11, 2001.
Christina Wyatt, MD, Associate Professor of Medicine (Nephrology), and Mary Ann McLaughlin, MD, MPH, Director of Cardiac Health for the Mount Sinai World Trade Center Health Program, are leading the investigation. Their research builds upon Dr. McLaughlin’s earlier study, WTC CHEST, which focused on the cardiac and pulmonary effects of particulate-matter exposure at the WTC site. Both studies are funded by the U.S. Centers for Disease Control and Prevention and the National Institute for Occupational Safety and Health.

The new study, WTC RENAL, will test the hypothesis that exposure to the dust cloud at Ground Zero caused inflammation of the vascular endothelium, increasing the risk of CKD in first responders. It will capitalize on the unique resources of the earlier study, including the standardized collection of data on particulate-matter exposure and shared risk factors for CKD and cardiovascular disease.
Drs. Wyatt and McLaughlin will measure spot urine albumin-to-creatinine ratios (UACR) as well as serum creatinine and serum cystatin C levels. Participants with abnormal results will be asked to return for confirmatory testing.
The prevalence of confirmed microalbuminuria and a decreased estimated glomerular filtration rate (eGFR) will be compared with nationally representative estimates, adjusting for age, gender, race, and traditional CKD risk factors. Both albuminuria and decreased eGFR are independently associated with increased cardiovascular risk.
The WTC RENAL study will take advantage of the unique opportunity to study these relationships in first responders, among whom preliminary data from the WTC CHEST study have already demonstrated an increased prevalence of diastolic dysfunction. The WTC RENAL study will explore whether abnormal UACR and eGFR are associated with increased left ventricular mass and diastolic dysfunction in first responders.
To explore potential mechanisms for kidney damage among first responders, a nested case-control analysis will compare established biomarkers of systemic inflammation, endothelial reactivity, and heavy metal exposure between WTC RENAL participants with albuminuria and/or decreased eGFR and a control group without evidence of kidney damage and with normal findings on echocardiography.
“Years after the tragic events of 9/11, many first responders who risked their lives to help others are facing the consequences of the toxic exposure at Ground Zero,” Dr. Wyatt says. “The WTC RENAL study will provide the best available evidence to determine whether kidney damage is one of those consequences.”
Detecting Fibrosis Before Damage to a Transplanted Kidney Is Irreversible
The roughly 17,000 individuals who receive kidney transplants each year in the United States face a sobering reality: 17 percent of those kidneys will fail by the third year, and by the tenth year fewer than half will still function. In most cases, the culprit is chronic fibrosis, which leads to severe tissue damage and kidney rejection.
While drug developers have been occupied with antifibrotic agents, Barbara Murphy, MD, the Murray M. Rosenberg Professor of Medicine at the Icahn School of Medicine at Mount Sinai, has been making steady progress in a less-crowded—but still critical—space. Dr. Murphy, who is also Chair of the Department of Medicine and Dean for Clinical Integration and Population Health, has created a portfolio of molecular assays to monitor kidney function both before and after transplantation to detect fibrotic events when it still may be possible to limit or prevent damage. These noninvasive assessments, known as FractalDx assays, rely on genetic signatures from simple blood tests to predict the likelihood of fibrosis and graft loss or rejection in transplant recipients.

Currently, measures of creatinine in the blood are used to monitor kidney status in transplant patients. The problem is that by the time increased creatinine levels are detected, fibrosis has already developed and the kidney has been irreversibly damaged. “Instead of being reactive and limiting the damage, we’re developing a monitoring tool that can identify problems before damage occurs,” Dr. Murphy explains. One assay, for example, can detect with high specificity an individual with inflammation in the kidney even if there has been no rise in creatinine. Another post-transplant assay is able to predict which individuals are at risk for fibrosis and likely to lose their grafts. In these cases, Dr. Murphy observes, “We could modify their immunosuppression, or add a therapeutic agent, which could prevent the onset of fibrosis and thereby protect the kidney and increase its longevity.”
Even more exciting to Dr. Murphy are the pretransplant baseline assays her lab is developing, which use recipient blood to stratify those at higher risk for acute rejection and fibrosis. Because this kind of assessment is not normally done, the majority of patients get a heavy regimen of three or four immunosuppressants at the time of the transplant. FractalDx assays, once developed and approved by regulators, she says, would provide a platform for “tailoring our therapies so that we maximize immunosuppression when a person is at risk, and minimize it when they’re not.”
Mining a Robust Pipeline
In keeping with its mission to ensure that its discoveries and innovations are translated into health care products and services, Mount Sinai has produced cutting-edge work in a wide range of laboratories. These advances include:
- The discovery of novel small molecules for treating lysosomal storage diseases (LSDs), a group of conditions caused by the most common genetic disorder in humans. In preclinical trials, these compounds have been shown to reverse the toxic lipid buildup in patients’ cells, which can severely damage tissue and organs.
- A new therapeutic approach to brain tumors that delivers autologous stem cells to the disease site at the time of tumor resection, bypassing the blood-brain barrier. These stem cells have been encoded with a tumor-cell-killer gene that is activated once the tumor returns, which is 95 percent of the time.
- A first-in-class small molecule that could bring new hope to individuals with the most severe cases of heart failure. The compound activates a protein known as SUMO1, which, in turn, stimulates the activity of SERCA2a, another protein responsible for the pumping action of the heart muscle.
- A novel way to treat Crohn’s disease through CEACAM5 peptides, which laboratory studies have shown activate CD8+ T-cells, reducing inflammation and restoring oral tolerance in the gut. CEACAM5 peptides would be among the first treatments to address the cause rather than the symptoms of Crohn’s disease.
Message from the Chief: John Ci-jiang He, MD, PhD
The Mount Sinai Health System Division of Nephrology is at the forefront of kidney care and science with expert-led research, cutting-edge clinical innovations, and state-of-the-art facilities.
 Mount Sinai’s Division of Nephrology houses the largest kidney clinical center in New York City, comprising 47 full-time faculty members, 5 acute hemodialysis centers, 10 chronic hemodialysis units, and one of the nation’s largest and most successful kidney-transplant programs. The peritoneal dialysis program is the largest in New York City and the second largest in New York State. The Mount Sinai Kidney Center launched a home hemodialysis program this year, providing patients with machines that are easy to use in the comfort and privacy of their homes.
Mount Sinai’s Division of Nephrology houses the largest kidney clinical center in New York City, comprising 47 full-time faculty members, 5 acute hemodialysis centers, 10 chronic hemodialysis units, and one of the nation’s largest and most successful kidney-transplant programs. The peritoneal dialysis program is the largest in New York City and the second largest in New York State. The Mount Sinai Kidney Center launched a home hemodialysis program this year, providing patients with machines that are easy to use in the comfort and privacy of their homes.
The year 2015 has been a tremendous one for the Mount Sinai Health System in providing and excelling in kidney care. The Mount Sinai Kidney Center on 117th Street in East Harlem, a brand-new dialysis center with state-of-the-art equipment, opened its doors to patients this past summer. The new facility encompasses more than 18,000 square feet of space and 36 stations, enabling the team to identify and treat more patients at the Center and in their own homes. It also includes an interventional suite to perform radiological procedures on-site.
Our physicians—many of whom have joined us from our large fellowship program, which recruits top trainees from prestigious residency programs—offer diverse clinical expertise focusing on viral kidney disease, diabetic nephropathy, kidney stones, and geriatric nephropathy. Our clinical expertise in these fields is complemented by a strong research program that is among the best funded in the country, receiving more than $10 million in National Institutes of Health (NIH) grants per year. The department’s NIH funding has increased significantly this year, with seven new R01 Research Project Grants. Our researchers, who conduct and support numerous clinical trials, have achieved international recognition as authorities on causes and treatments of all forms of kidney diseases and disorders—one reason that U.S. News & World Report this year ranked The Mount Sinai Hospital No. 23 in the nation for nephrology.
John Ci-jiang He, MD, PhD, is Chief of the Division of Nephrology, the Irene and Dr. Arthur M. Fishberg Professor of Medicine, and Professor of Pharmacology and Systems Therapeutics.
Division of Nephrology
Download the Winter 2016 Specialty Report