Immunotherapy
Mount Sinai is a leader in immunotherapy research and clinical applications for cancer.
Immunotherapy uses the power of your immune system to attack cancer. It has become a significant component of cancer treatment and is bringing breakthrough treatments to increasing numbers of patients for improved outcomes and sustained remissions.
A New Approach to Immunotherapy: How CAR T Cells Help Fight Cancer
As cancer progresses, your immune system may become more and more compromised. This can contribute to rapid tumor growth. Our Cancer Immunology Research Program identifies what makes it difficult for the immune system to defend itself against tumors. Once we identify these mechanisms, we can better develop novel immunotherapies to kill cancer cells.
Adoptive Cell Therapy (ACT)
We use an advanced form of immunotherapy called adoptive cell therapy (ACT) to treat cancer. ACT uses your own T cells (immune cells) to treat your cancer. CAR T-cell therapy, a type of adoptive cell transfer, has dramatically helped some patients who have not benefited from other treatments.
The Living Drug
When we use CAR T-cell therapy, we start by drawing blood and separating out the T cells. Then we genetically engineer the T cells to produce receptors on their surface called chimeric antigen receptors (CARs). These receptors enable the T cells to recognize and attach to a specific protein, or antigen, on cancer cells. Once these cells can express the antigen-specific CAR, we expand them, in the laboratory, into the hundreds of millions. Finally, following a chemotherapy regimen, we infuse the CAR T cells back into you. The engineered CAR T cells function as a living drug that is designed to further multiply in your body and recognize and kill cancer cells that contain the antigen on their surfaces.
This video from the National Cancer Institute (NCI) explains how immunotherapy works.
Immunotherapy Clinical Trials
We use CAR T-cell and innovative immunotherapies to treat various cancers through the Cellular Therapy Service.
We were among the first institutions to participate in clinical trials that use CAR T cells for multiple myeloma. Our experience began with Celgene’s Phase 1 bb2121 CRB-401 dose escalation and dose expansion study. Mount Sinai was one of only nine sites participating in this trailblazing CAR T-cell study. Results were reported in the New England Journal of Medicine.
Immunotherapy Clinical Trials for Newly Diagnosed Myeloma Open to Patient Enrollment
- A Phase 3 Randomized Study Comparing Daratumumab, Bortezomib, Lenalidomide and Dexamethasone (DVRd) followed by Ciltacabtagene Autoleucel versus Daratumumab, Bortezomib, Lenalidomide and Dexamethasone (DVRd) followed by Autologous Stem Cell Transplant (ASCT) in Participants with Newly Diagnosed Multiple Myeloma who are Transplant Eligible, Mount Sinai Protocol #23-01451, https://clinicaltrials.gov/ct2/show/NCT05257083
Immunotherapy Clinical Trials for Relapse/Refractory Myeloma Open to Patient Enrollment
CAR T-cell Therapy
- A Phase 2, Multi-Cohort, Open-Label Multicenter Study to Evaluate the Efficacy and Safety of bb2121 in Subjects with Relapsed and Refractory Multiple Myeloma and in Subjects with Clinical High-Risk Multiple Myeloma, GCO# 40-5076, ClinicalTrials.gov Identifier: NCT03601078
- A Phase 2, Multicohort Open-Label Study of JNJ-68284528, a Chimeric Antigen Receptor T cell (CAR-T) Therapy Directed Against BCMA in Subjects with Multiple Myeloma, GCO 40-5112, ClinicalTrials.gov Identifier: NCT04133636
- A Phase 1, Multicenter, Open-Label Study of CB-011, a CRISPR-Edited Allogeneic Anti-BCMA CAR-T Cell Therapy in Patients with Relapsed/Refractory Multiple Myeloma (CAMMOUFLAGE Trial), Mount Sinai Protocol #22-2063, https://clinicaltrials.gov/ct2/show/NCT05722418
- A Phase 1b/2 Study of GC012F, a Chimeric Antigen Receptor T-cell (CAR T) Therapy Targeting CD19 and B-cell Maturation Antigen (BCMA) in Subjects with Relapsed/Refractory Multiple Myeloma, Mount Sinai Protocol #23-0881, https://clinicaltrials.gov/ct2/show/NCT05850234
- A Phase 1, Open-Label, Dose-Finding Study of BMS-986453, Dual Targeting BCMAxGPRC5D Chimeric Antigen Receptor T Cells, in Participants with Relapsed and/or Refractory Multiple Myeloma, Mount Sinai Protocol #23-01500, https://clinicaltrials.gov/ct2/show/NCT06153251
- A Phase 2, Open-Label, Multicenter Study of BMS-986393, a GPRC5D-directed CAR T Cell Therapy in Adult Participants with Relapsed or Refractory Multiple Myeloma (QUINTESSENTIAL), Mount Sinai Protocol #23-01591, https://clinicaltrials.gov/ct2/show/NCT06297226
Antibody Therapy
- Phase 1, First-in-Human, Dose Escalation Study of JNJ-79635322, a Trispecific Antibody, in Participants with Relapsed or Refractory Multiple Myeloma or Previously Treated AL Amyloidosis, Mount Sinai Protocol #24-00084, https://clinicaltrials.gov/ct2/show/NCT06083922
- A Dose Escalation and Expansion Study of ABBV-383 in Combination with Anti-Cancer Regimens for the Treatment of Patients with Relapsed/Refractory Multiple Myeloma, Mount Sinai Protocol #40-5236, https://clinicaltrials.gov/ct2/show/NCT05259839
- A Phase I/II, Open-Label, Multi-Comulti-Cohort Study to Evaluate the Efficacy and Safety of Cevostamab in Prior B Cell Maturation Antigen-Exposed Patients With Relapsed/Refractory Multiple Myeloma, Mount Sinai Protocol #22-2046, https://clinicaltrials.gov/ct2/show/NCT05535244
For information about immunotherapy clinical trials for multiple myeloma, contact MMLEADERSHIP@mssm.edu.
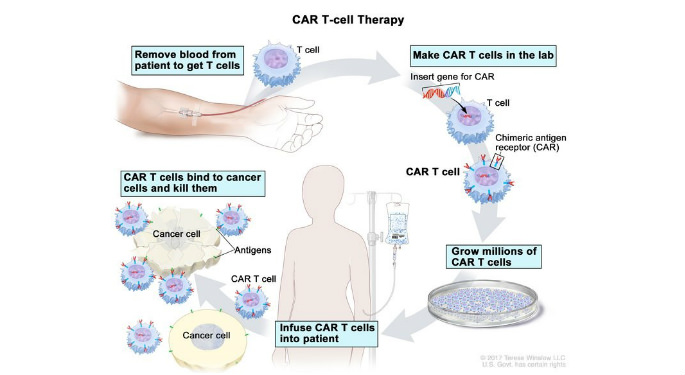
Blood is removed from a vein in a patient’s arm to get T cells; a special receptor called a chimeric antigen receptor (CAR) is made in the laboratory; the gene for CAR is inserted into the T cells and then millions of CAR T cells are grown; CAR T cells are given to the patient by infusion (after chemotherapy); the CAR T cells bind to antigens on the cancer cells and kill them.
Image Source: National Cancer Institute
