Visitor Policies
We welcome visitors with restrictions, before you visit please see our latest policy
Coronavirus (COVID-19) Facts and Resources
COVID-19 is an illness caused by a coronavirus, a kind of virus that causes symptoms that feel like a cold, the flu, or pneumonia. The virus is spread when an infected person releases droplets through their nose or mouth while breathing, speaking, coughing, or sneezing. Some infected people have no symptoms or mild symptoms but can still spread the virus.
Older adults and people with certain medical issues are at greater risk for serious disease and death. People who are unvaccinated are also at higher risk. Some people, including those who have no symptoms or mild symptoms, may still develop “long COVID,” meaning they continue to experience health issues months after their infection.
Symptoms and Prevention
Mild COVID-19 symptoms often mimic a cold, with symptoms that include shortness of breath or difficulty breathing. In addition to the heart and lungs, COVID-19 can affect other parts of the body; other symptoms may include new loss of taste or smell, nausea, and diarrhea.
The best way to protect yourself and others from COVID-19 is to get vaccinated, including the most up-to-date shot. Please check with your health care provider or local pharmacy to get the most up-to-date shots. Patients ages 16 to 18 need parental/guardian consent for vaccination. Patients 15 and under must be accompanied by a parent or guardian.
Other ways to protect yourself or others are to wear a mask in crowded spaces when transmission in the community is high, wash your hands often with soap for at least 20 seconds, and take a test if you think you could be infected. To get tested, purchase an at-home antigen test at your local pharmacy or visit one of our Mount Sinai Urgent Care locations. If your test is positive, you may schedule a follow-up virtual or in-person visit with a health care provider.
Treatments for COVID-19
Most people who are up to date with their COVID-19 vaccine do not need treatment. However, high-risk individuals, including those who are immunocompromised, have underlying health conditions, and are unvaccinated may benefit from taking antiviral treatment.
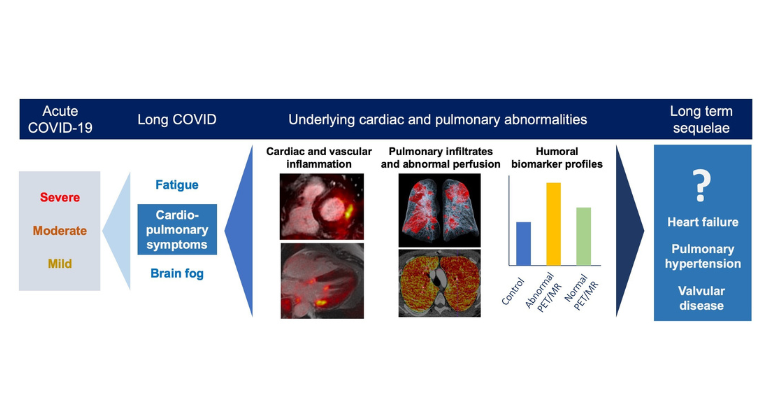
Information About Long COVID
Some people who begin to recover from COVID-19 continue to experience heart issues, shortness of breath, fatigue, or cognitive difficulties—often for weeks or months. This has become known as long COVID. Mount Sinai founded the Center for Post-COVID Care to help these patients, offering services at three sites. For more information or to contact the Center, please view its web page.
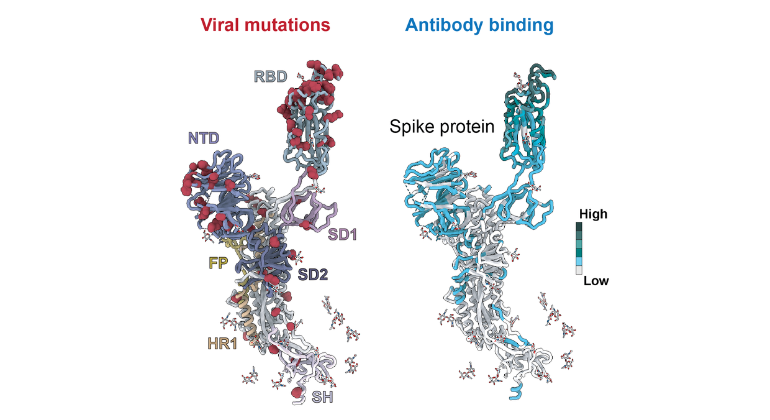
Scientists Uncover How COVID-19 Variants Outsmart the Immune System
Nov 21, 2025 View All Press Releases