
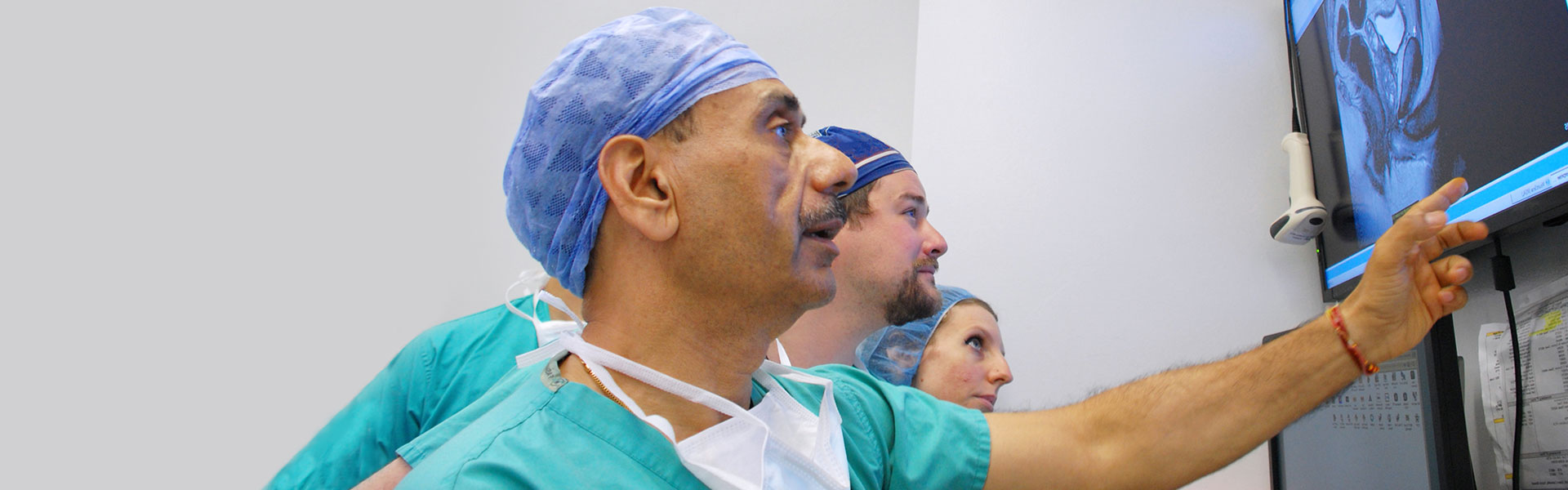
A Leader in Urologic Care
The Mount Sinai Hospital is ranked No. 6 in the nation for Urology by U.S. News & World Report® for 2025-26.

Urology Specialty Care at Mount Sinai Ranks Among Best in World
Newsweek Names The Mount Sinai Hospital Among the Highest Quality Internationally for Urology

PAs at the Frontline
Learn more about our Urology physician assistants sharing their journey.

Get Screened For Prostate Cancer
It’s important to get screened regularly for prostate cancer—and our mobile prostate unit comes to neighborhoods near you
Urology Department Brochures
See brochures that highlight our treatment and research of prostate cancer, kidney cancer, bladder cancer, and kidney stones.

Innovation in the field of robotic prostate cancer surgery
Cultivating the brightest minds to bring you the best care

Minimally Invasive Treatments and Surgery
For urologic conditions including bladder, kidney and prostate cancer
Urology

Urology
The Milton and Carroll Petrie Department of Urology is part of the Mount Sinai Health System. The Department of Urology has been a leader in clinical practice, research and education since 1852, with the establishment of the Mount Sinai Hospital. Today, depth of knowledge, breadth of experience and compassion for patients continue to characterize our highly respected team of board certified and fellowship trained physicians who are on the faculty of the Icahn School of Medicine at Mount Sinai. We have over 190 urologists on staff – more than any NYC healthcare system. The Mount Sinai Hospital is ranked No. 6 in the nation for Urology by U.S. News & World Report® for 2025-26.
Our surgeons and scientists have pioneered the adoption of novel diagnostic techniques and minimally invasive treatments for prostate, kidney and bladder cancers and a wide range of urologic disorders.
Our experts are among the most renowned in the world performing robotic surgery; our robotics program is among the most robust in the country. Our Kidney Stone Center was the first of its kind in New York, focusing on both treatment and prevention. Genomic testing and advanced imaging are routine for patients with prostate cancer in order to provide them with the most personalized and precise treatment protocols. Our specialized men’s, women’s, children’s, reconstructive and transgender programs ensure that we can meet the needs of every patient with exceptional quality of service.
For more information or to schedule an appointment, please call 212-241-9955.
Urology at Mount Sinai Health System


Robotic Prostate Surgery
Enhancing patient results with minimally invasive procedures in robotic prostate surgery
Learn More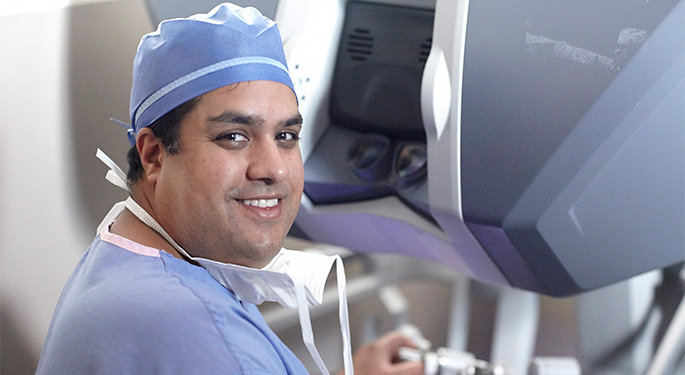
Robotic Kidney and Reconstructive Surgery
Treating kidney cancer with minimally invasive procedures in robotic kidney surgery
Learn More
Kidney Stones Center
Providing expert care in managing and treating kidney stones of all sizes
Learn More
Men’s Health
Treating male incontinence, infertility and erectile dysfunction with experience and sensitivity
Learn More
Minimally Invasive Surgery
Leading the area of minimally invasive surgical procedures with unparalleled patient outcomes
Learn More
Reconstructive Urology
Providing you with comprehensive, individualized examinations and treatment options
Learn More
Focal Therapy and Interventional Urology
Combining the skills of interventional radiologists with new imaging technologies to improve care
Learn More
Integrative Urology and Wellness
Offering the best in holistic, integrative medicine to men and women with genitourinary tract disorders
Learn More
Urologic Cancers
Pioneering the treatment of adrenal, bladder, kidney, prostate, and testicular cancers
Learn More
Urinary Incontinence
Diagnosing and treating various types of urinary incontinence, including urethral stricture
Learn More
Women’s Urology
Providing expert care for female incontinence, pelvic organ prolapse, bladder fistulas, and other urogynecologic disorders
Learn More-
The Mount Sinai Hospital
5 East 98th Street, 6th Floor New York, NY 10029
Phone:
-
Mount Sinai Doctors - 425 West 59th Street
425 West 59th Street, Suite 4F New York, NY 10019
Phone:
-
Mount Sinai Morningside
440 West 114th Street, Suite 640 New York, NY 10025
Phone:
-
The Mount Sinai Hospital
5 East 98th Street, 6th Floor New York, NY 10029
Phone:
-
Mount Sinai Morningside
440 West 114th Street, Suite 640 New York, NY 10025
Phone:
-
Mount Sinai Doctors - 425 West 59th Street
425 West 59th Street, Suite 4F New York, NY 10019
Phone: