Center to Find Drug Combinations that Reduce Side Effects
A research team from the Icahn School of Medicine at Mount Sinai today received a $12 million grant from the National Institutes of Health to create a center that will screen massive data sets for new uses of existing drugs, and confirm them in human cell tests. The center’s first mission will be to find FDA-approved drugs that reduce side effects when paired with hundreds of leading drugs against common, deadly diseases.
With advances in inexpensive computing power, and stored data collections becoming truly massive in the era of “big data,” researchers are just now able to design algorithms and models that pull previously unrecognized disease and drug treatment patterns from databases. These computational patterns are predictive, and researchers can validate them with experiments.
“The goal of our new center is to detect changes made in human heart, liver and nerve cells as otherwise useful drugs cause side effects, and to find the combinations of existing drugs that reduce these side effects,” said Ravi Iyengar, PhD, the Dorothy H. and Lewis Rosenstiel Professor in the Department of Pharmacology and Systems Therapeutics within the Icahn School of Medicine at Mount Sinai, and the lead investigator for the center grant.
“Our center embodies a third way to reduce the side effects that limit the use of so many treatments, along with two traditional approaches: fine-tuning a drug’s chemical structure or tailoring its use for each individual’s genetics,” he added.
Hidden Signatures
The new grant will fund a Drug Toxicity Signature Generation Center at Mount Sinai as part the NIH Common Fund’s LINCS program, the Library of Integrated Network-based Cellular Signatures program. Each signature is a confirmed set of genetic and protein responses within a type of cell to a drug or drug combination.
The team will find such signatures by combining high-throughput experiments on cell responses to drugs with statistical analyses of side effect, gene and protein interaction databases. Interestingly, the team starts with stem cells and then converts them into the heart, liver and nerve cells used in the experiments. The new center’s goal is to generate 2,000 signatures per year for further testing.
To anchor the signatures to human diseases and treatments, the team will search the U.S. Food and Drug Administration’s Adverse Event Reporting System (FAERS) database to find cases where adding a second drug reduced the side effects associated with a commonly used primary treatment. FAERS has for decades collected such data from individuals, health professionals, drug companies and hospitals, and the millions of records on patients taking multiple drugs now in this public database are free and open to all researchers for analysis.
To translate FAERS-generated drug combinations that reduce toxicity into networks of mechanism-based cell response signatures, the team will then run the experimental results through other databases, including NIH databases of human DNA sequences and interactions between proteins. These networks will be filtered using sophisticated modeling techniques to increase the reliability of the signatures. The most promising signatures can then form the basis for targeted animal and human clinical studies on “drug repurposing.”
The new center will focus on finding drug combinations which reduce side effects that affect the heart (cardiotoxicity), liver (hepatotoxicity) or nervous system (peripheral neuropathy).
“These areas represent the most common and dose-limiting side effects that come with many first-line treatments for heart disease, cancer and diabetes, for instance,” said Dr. Iyengar, who is also Director of the Systems Biology Center New York within the Icahn School of Medicine at Mount Sinai. “While still in the early stages, our efforts have the potential to find second drugs that reduce side effects with a dramatic impact on human health, and in another step toward personalized, precision medicine.”
Along with Dr. Iyengar, the principal investigators for the grant are Marc Birtwistle PhD, Assistant Professor, and Eric Sobie, PhD, Associate Professor, both in the Department of Pharmacology and Systems Therapeutics at Mount Sinai. Christoph Schaniel, PhD, also Assistant Professor in the Department, will lead the efforts to generate heart, liver and nerve cells from human induced pluripotent stem cells taken from normal subjects (healthy organs).
Other Mount Sinai investigators behind the successful grant submission were Milind Mahajan, PhD, in the Department of Genetic and Genomic Sciences and Director of the Genomics Core, James Gallo, PhD, and Avner Schlessinger, PhD, both also in the Department of Pharmacology and Systems Therapeutics. Hong Li, PhD, Director of the Center for Advanced Proteomics at Rutgers New Jersey Medical School will oversee proteomics within the new center.
This center grant is being funded by the NIH Common Fund, which was created by Congress in 2006 to overcome roadblocks in research by funding bold, cross-disciplinary projects with the potential to make leaps and greatly improve human health. Mount Sinai’s center grant will be $2 million per year over the next six years.
The Common Fund supports the LINCS program, which seeks to create a better understanding of human physiology by using a “systems” approach to cataloging changes that occur when cells are exposed to a variety of “perturbing agents,” or agents that change cell function.
About the Mount Sinai Health System
Mount Sinai Health System is one of the largest academic medical systems in the New York metro area, with more than 43,000 employees working across eight hospitals, over 400 outpatient practices, nearly 300 labs, a school of nursing, and a leading school of medicine and graduate education. Mount Sinai advances health for all people, everywhere, by taking on the most complex health care challenges of our time — discovering and applying new scientific learning and knowledge; developing safer, more effective treatments; educating the next generation of medical leaders and innovators; and supporting local communities by delivering high-quality care to all who need it.
Through the integration of its hospitals, labs, and schools, Mount Sinai offers comprehensive health care solutions from birth through geriatrics, leveraging innovative approaches such as artificial intelligence and informatics while keeping patients’ medical and emotional needs at the center of all treatment. The Health System includes approximately 7,300 primary and specialty care physicians; 13 joint-venture outpatient surgery centers throughout the five boroughs of New York City, Westchester, Long Island, and Florida; and more than 30 affiliated community health centers. We are consistently ranked by U.S. News & World Report's Best Hospitals, receiving high "Honor Roll" status, and are highly ranked: No. 1 in Geriatrics and top 20 in Cardiology/Heart Surgery, Diabetes/Endocrinology, Gastroenterology/GI Surgery, Neurology/Neurosurgery, Orthopedics, Pulmonology/Lung Surgery, Rehabilitation, and Urology. New York Eye and Ear Infirmary of Mount Sinai is ranked No. 12 in Ophthalmology. U.S. News & World Report’s “Best Children’s Hospitals” ranks Mount Sinai Kravis Children's Hospital among the country’s best in several pediatric specialties.
For more information, visit https://www.mountsinai.org or find Mount Sinai on Facebook, Twitter and YouTube.
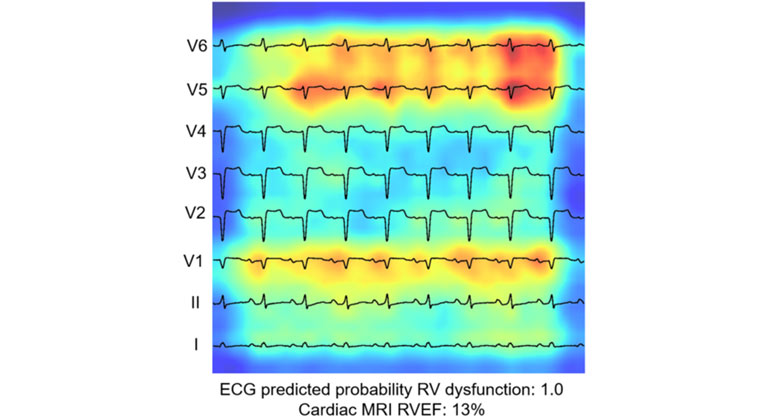
AI-Driven Study Redefines Right Heart Health Assessment With Novel Predictive Model
Jan 04, 2024 View All Press Releases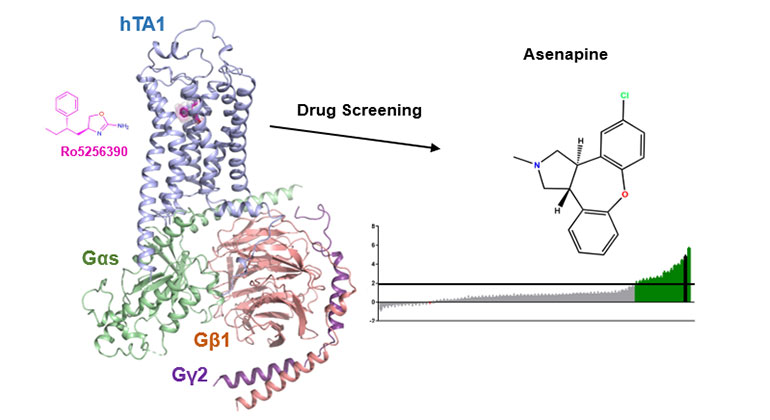
Demystifying a Key Receptor in Substance Use and Neuropsychiatric Disorders
Jan 02, 2024 View All Press Releases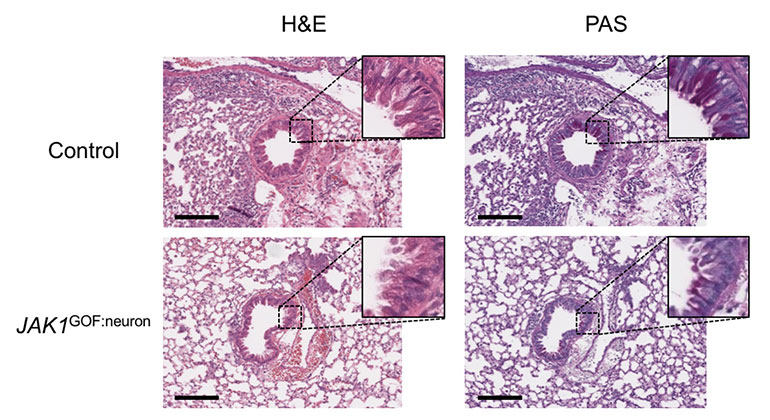
New Insights Revealed On Tissue-Dependent Roles of JAK Signaling in Inflammation
Dec 21, 2023 View All Press Releases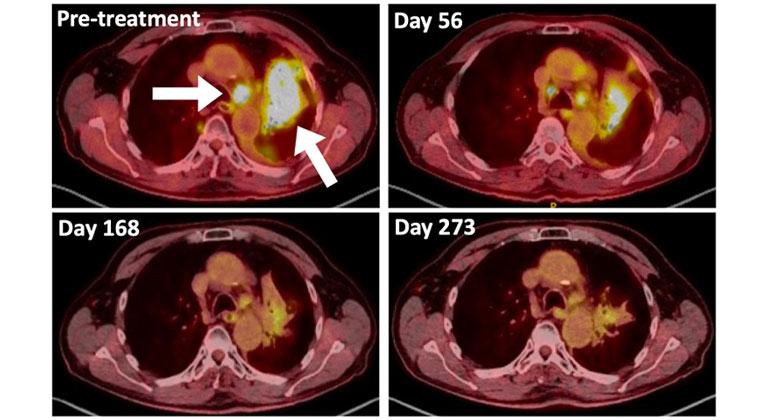
A Type of Allergy Medicine Might Help Treat Lung Cancer, Research Suggests
Dec 06, 2023 View All Press Releases